This article has been
cited by other articles in ScienceCentral.
Abstract
Gastroesophageal reflux disease (GERD) is closely related to respiratory issues. We reported the case about the nutrition intervention given to a male infant with congenital bronchomalacia, GERD, and recurrent pneumonia. During the first and second pediatric intensive care unit (PICU) stays, his nutrition status and nutrient intake were good. However, during the 18 days of the third PICU admission, his nutrient intake decreased to 75%–80% of his estimated calorie requirement and his Z-score for weight-for-age dropped to −1.4. We conducted nutritional interventions to improve GERD symptoms and nutritional status include avoiding overfeeding by feeding small amounts frequently, using a pre-thickened formula mixed with a high-calorie formula, and feeding through transpyloric tube. As a result, his daily nutrient intakes gradually increased and his Z-score for weight-for-age was normal. In conclusion, it is important to implement individualized intensive nutritional management to ensure adequate nutrition and growth status in infants with lung disease and GERD.
Keywords: GERD, Pneumonia, Enteral nutrition, Infant
INTRODUCTION
Gastroesophageal reflux (GER) is common in infants. Physiologic GER without any symptoms other than regurgitation and vomiting is typically resolved within one or two years without medical treatment. In case of gastroesophageal reflux disease (GERD) accompanied by pathological symptoms such as failure to thrive, apnea, or aspiration, the patient must be evaluated and treated according to the diagnosis [
1].
Children with GERD should be provided with adequate nutrition to ensure normal growth while receiving pharmacological and surgical treatment. Nutrition intervention strategies to improve GERD symptoms include feeding the patient smaller and more frequent meals, using pre-thickened infant formulas or hydrolyzed formulas for those with milk protein allergies and doing so through transpyloric or jejunal feeding [
12].
This case is about the nutrition intervention given to an infant experiencing recurrent pneumonia associated with congenital broncholmalacia and GERD. The progression of the clinical course and nutrition intervention of the patient during hospitalization was shown in
Figure 1. This study was approved by the Institutional Review Board in Severance Hospital (approval No. 4-2019-0769).
Figure 1
Summary of the patient's clinical course and nutrition intervention.
PICU, pediatric intensive care unit; HOD, hospital of day; GER, gastroesophageal reflux; PN, parenteral nutrition; GI, gastrointestinal; VFSS, videofluoroscopic swallowing study.

CASE
A male infant born weighing 3,380 g during the 38th week of intrauterine pregnancy was admitted to the pediatric cardiac care unit (PCCU). He was treated for respiratory distress syndrome for the first 13 days of his life in the neonatal intensive care unit in another hospital. At 23 days old, he was readmitted to the same hospital due to cyanosis with crying. On his echocardiogram, patent ductus arteriosus and tricuspid regurgitation was detected. On the 27th day after birth, he was transferred to our PCCU for cardiac evaluation. However, his cardiac evaluation in our hospital did not reveal any problems. He was transferred to the general ward on the 16th day of hospitalization in our hospital after receiving lung care for pneumonia. He was transferred to the pediatric respiratory allergy department for active treatment of pneumonia. After that, he was admitted to the PICU twice and was treated with mechanical ventilation due to recurrent pneumonia. During the first PICU stay, an electroencephalogram and an echocardiogram were performed to identify the reason for the recurrent pneumonia, but they revealed a low likelihood of central apnea or seizure and no cardiac problem. During the 24-hour esophageal pH monitoring, his reflux index was 0.9% and DeMeester composite score was 2.9, indicating that he had physiologic GER. During the second PICU stay, an esophagography was performed to rule out the existence of trachoesophageal fistula and revealed no sign of fistula or gastroesophageal reflux. Fiberoptic bronchoscopy (FOB) revealed severe bronchomalacia on the right main bronchus and left lower lobe bronchus. He exhibited slight wheezing and dyspnea during bottle feeding before the second PICU admission, so a videofluoroscopic swallowing study (VFSS) was performed to evaluate his swallowing function and aspiration risk during bottle feeding. The rehabilitation department recommended tube feeding. During the first and second PICU stays, he was referred to the pediatric nutrition support team (PNST). His nutritional status and nutrient intake were good.
On the 52nd day of hospitalization, he was admitted for the third time to the PICU due to respiratory difficulty and received mechanical ventilation therapy for approximately five weeks. PNST consultation was conducted 7 times throughout this PICU stay. Changes in his calorie intake and protein intake through enteral nutrition and parenteral nutrition were shown in
Figure 2 and
3. His Z-score for weight-for-age over the course of the management is shown in
Figure 4. His initial weight for his age group was in the 10–25th percentile and his Z-score for weight-for-age was −0.95, both within the normal range. However, during the 18 days of PICU admission, his nutrient intake was 82 kcal/kg, which was 75%–80% of his estimated calorie requirement. His Z-score for weight-for-age gradually dropped to −1.4 (
Figure 4). Feeding difficulties due to desaturation and chest retraction and frequent fasting for testing and treatment procedures, such as prone positioning, weaning from the ventilator, and performing chest computed tomography, hampered adequate nutrient intake.
Figure 2
Changes in energy intake through EN and PN.
EN, enteral nutrition; PN, parenteral nutrition; HOD, hospital of day.
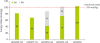
Figure 3
Change in protein intake through EN and PN.
EN, enteral nutrition; PN, parenteral nutrition; HOD, hospital of day.

Figure 4
The patient's Z-score for weight-for-age over the course of the nutritional management.
HOD, hospital of day.

PNST was consulted at this time. Increased nutrient intake was mandated for weight gain. However, increased feeding volume was not recommended due to recurrent aspiration pneumonia. High-calorie infant formula of 1 kcal/mL mixed with infant formula increased the calorie density to 0.79 kcal/mL and allowed for a reduction in feeding volume from 110 mL/feed to 100 mL/feed. Feeding rate was reduced to 100 mL/hr using a feeding pump in order to reduce intra-gastric pressure and the risk of regurgitation. As a result, calorie intake increased to 97 kcal/kg/day from the following day. However, he fasted for two days for prone positioning due to an aggravation of his lung condition. Tube feeding was restarted, but he vomited three times after coughing. The location of the feeding tube tip was moved to the post-pyloric portion of the jejunum with blind technique at bedside to reduce the risk of aspiration. After this procedure, the location of the tube tip was confirmed by abdominal X-ray. Feeding was tolerable for 7 days. When tube feeding was insufficient due to procedures, such as prone positioning, weaning from the ventilator, and FOB, supplementary parenteral nutrition (PN) was added. Average calorie intake via tube feeding and PN was 89 kcal/kg/day, 90% of his estimated requirement.
Extubation was performed the 37th day of PICU stay, the 88th day of hospitalization. However, he exhibited hardship while feeding. Medical staff decided to re-evaluate the GERD diagnosis. During the second 24-hour esophageal pH monitoring, his reflux index was 19.5% and DeMeester composite score was 70.4, both of which were worse than the previous results from 2 months prior. The gastroenterologist recommended an increase in the Proton pump inhibitors (PPIs) dose and the use of the pre-thickened formula (Novalac AR®).
Novalac AR® formula replaces a portion of carbohydrates with cornstarch to increase the formula's viscosity when it comes into contact with gastric acid. This formula is thicker than standard infant formulas and can reduce the number of incidences of regurgitation and vomiting. However, it was thought that reduced secretion of gastric acid caused by increasing the PPIs dose might interfere with the effects of this formula. This formula has a lower calorie density than standard infant formulas at 0.66 kcal/mL, so the PNST dietitian recommended mixing the high-calorie formula of 1 kcal/mL with this formula to increase the formula's calorie density to 75 kcal/100 mL. He tolerated the mixed formula and maintained a proper calorie intake of 85–95 kcal/kg/day, 90% of his estimated requirement.
No aspiration was observed during the VFSS conducted to support his translation to an oral diet. As a result, it was decided that bottle feeding could be tried with oral motor facilitation to promote the oral phase of swallowing. On the 105th day of hospitalization, he was transferred to the general ward. Bottle feeding with a small volume of formula began on the 110th day of hospitalization. The PNST dietitian monitored the nutrient intake from tube feeding and bottle feeding. On the 117th day of hospitalization, calorie intake from bottle feeding was sufficient to meet 90% of his estimated requirement and the feeding tube was removed.
Before discharge, his calorie intake from bottle feeding was 104 kcal/kg/day, his weight was in the 10–25th percentile for his age, and his Z-score for weight-for-age was −0.8, all of which were normal.
DISCUSSION
GERD is closely related to respiratory issues. Respiratory illnesses lead to an increase in inspiratory effort, causing a rise in abdominal pressure. Together, these conditions can exacerbate GERD symptoms [
3]. Mechanical ventilation can weaken the diaphragm, affect the defensive reflex mechanisms of the upper trachea and the sphincter muscles of the upper esophagus, along with reflux-induced aspiration, and thus worsen respiratory illnesses [
4]. While these conditions further prevent the supply of sufficient nutrients, they also place greater stress on breathing effort, thus increasing energy demands. Since this can result in poor growth or developmental disorders, it is crucial to administer clinical treatments and constant nutritional management.
During the 18 days of PICU admission, the infant's nutritional status was aggravated and was related to inadequate nutrient intake. His feeding volume per feeding was reduced (110 mL/feed → 100 mL/feed), and calorie intake per feeding was increased by using the high-calorie infant formula mixed with standard formula (67 kcal/mL [14%] → 79 kcal/mL [16.5%]). As outlined in the 2018 North American Society For Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and European Society For Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) pediatric GERD practice guidelines, overfeeding infants showing suspected symptoms of GERD must be avoided through non-pharmacologic interventions, thickened feeding is a highly recommended approach [
1]. Although no randomized controlled trials have been conducted on the effects of avoiding overfeeding by feeding small amounts frequently, this method is strongly recommended in many reviews and guidelines. These recommendations suggest determining the appropriate total daily amount of formula by adjusting feeding frequency and volume for age and weight. This approach should be attempted first over medicinal or surgical interventions, as it bears fewer risks and a smaller financial burden [
1]. Also, considering that a relatively higher feeding rate increases pressure within the stomach and decreases pressure in the lower-esophageal sphincter, thus leading to increased reflux, it is beneficial to supply the nutrients to the patient slowly [
15].
We applied a feeding pump to provide each feeding slowly over the course of one hour, resulting in improved compliance.
The patient in this case study underwent 24-hour esophageal pH monitoring and exhibited symptoms indicating worsening GER; therefore, the gastroenterologist recommended administering Novalac AR
® and increasing the PPIs dose. The pre-thickened formula increases viscosity upon interaction with gastric acid. Many studies showed that pre-thickened formulas are effective in reducing visible regurgitation and vomiting, while their impact on gastric emptying was not significantly different from that exhibited by standard infant formulas [
15]. However, there is a lack of evidence regarding the use of pre-thickened formulas as a treatment method for improving reflux index during the 24-hour esophageal pH monitoring [
67]. Particular precautions need to be taken when administering pre-thickened formulas to premature infants, as they can result in inadequate supplies of nutrients, such as protein, calcium, and phosphorous, as well as in decreased absorption levels in the intestines [
8]. Also, this formula is characterized as naturally having a greater viscosity than standard infant formulas, with the viscosity increasing with time. These qualities increase sucking effort when bottle feeding, while posing a risk of blockage when tube feeding, thus making it difficult to supply concentrated amounts [
8]. As a result, special attention must be taken to provide adequate energy in concentrated amounts when using this formula or any similar formula. In this study, Novalac AR
® (0.66 kcal/mL) formula was not concentrated, but rather mixed with a high-calorie formula to increase its total caloric density (0.75 kcal/mL) in order to meet the patient's increased nutrient requirement. Additionally, he was administered more PPIs, so it was expected that the resulting decrease in gastric acid secretion would have a negative influence on the viscosity of this formula.
Moreover, in this case, the end of the tube was inserted through the pyloric sphincter to reduce the risk of aspiration for the patient. Some studies showed that administering transpyloric tube feeding might be useful in reducing the risk of reflux-related pneumonia, as well as in reducing instances of reflux-related apnea and bradycardia in premature infants [
59]. However, this method must be accompanied by close monitoring and management, as it increases the risk of complications such as tube blockage or displacement, gastrointestinal intussusception, and intestinal perforations. Over the course of 17 days, transpyloric tube feeding was tolerated without vomiting. Supplementary PN was added when tube feeding was insufficient due to medical procedure. Average calorie intake via tube feeding and PN was 89 kcal/kg/day, which is 90% of his estimated requirement.
According to the 2018 NASPGHAN and ESPGHAN practice guidelines, surgical treatments are recommended for patients with refractory GERD symptoms who do not respond to non-pharmacological and pharmacological treatment methods [
1]. For the patient in this case study, fundoplication was recommended by the designated physician, but his parents did not agree with having the surgical procedure. Despite the fact that this medical condition requires surgical indications, there are many instances where such procedures are not implemented at the appropriate time due to negative perceptions or delays while the patient's parents arrive at a decision [
10]. As a result, patients exhibit worsening malnutrition due to inadequate nutrient intake in many of these cases. For instances where the decision to undergo surgical treatment is delayed, it is important to implement the proper clinical treatment and nutritional support to ensure adequate nutrition and growth status.
In conclusion, it seems that this individualized nutrition support has a positive influence on the adequate nutrition and growth of infants with lung disease and GERD.












