This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Mycobacterium terrae complex (MTC) are slow-growing nontuberculous mycobacteria (NTM) that cause tenosynovitis, septic arthritis, and osteomyelitis of the extremities [
1]. Eleven new species of MTC have been described since 2006 [
2].
Mycobacterium virginiense, a member of the MTC, was first described in 2016, and isolates from patients with tenosynovitis and osteomyelitis are acid-fast, slow-growing, and nonpigmented on Middle brook 7H10 agar [
2]. The first case of
M. virginiense isolated from a human pulmonary specimen was reported in 2019 [
3]. We report the first case of tenosynovitis caused by
M. virginiense in Korea. This retrospective study was approved by the Institutional Review Board of Jeju National University Hospital, Jeju, Korea (No. 2019-07-018) that waived informed consent.
A previously healthy 68-year-old woman was admitted to Jeju National University Hospital with painful swelling of the third finger of her left hand, which had been present for one month; her hand was treated with acupuncture two months before admission. She had a leukocyte count of 5.70 (reference range: 4.5–11.0)×10
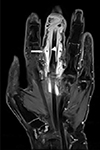
9/L, an erythrocyte sedimentation rate of 41 (reference range: 0–20) mm/hr, and C reactive protein levels of 2.10 (reference range: 0.76–28.5) nmol/L. Magnetic resonance imaging of her left hand revealed an effusion, synovial proliferation of the flexor tendon sheath, and subcutaneous fat edema of the third finger (
Fig. 1). She was treated empirically with cefazolin and clindamycin for three weeks following which she underwent surgical debridement. Tenosynovitis and severe inflammatory changes of the soft tissue surrounding the flexor tendon were observed during surgery. Bacterial culture, acid-fast bacilli (AFB) stain, and tuberculosis (TB)-PCR were negative in the tissue specimen of the inflammatory soft tissue surrounding the tendon. On day 22, an AFB culture performed at the Korean Institute of Tuberculosis revealed an unidentified NTM. Cefazolin and clindamycin were discontinued, and oral clarithromycin (500 mg twice a day) and intravenous amikacin (10 mg/kg/day) and cefoxitin (12 g/day) were started, based on a presumptive diagnosis of rapid-growing NTM infection. Two weeks later, the regimen was changed to oral clarithromycin and ciprofloxacin (500 mg twice a day). The patient improved clinically and was discharged on hospital day 40.
Molecular methods, including
rpoB gene PCR, were used for definitive species identification, and slow-growing
M. virginiense was confirmed with an accuracy of 99% (341/343 bp) using the basic local alignment search tool algorithm (
Fig. 2) [
4]. The
rpoB gene sequences were aligned using the multiple-alignment algorithm in the MegAlign program (Windows version 3.12e; DNASTAR, Madison, WI, USA) and the ClustalX program (Toby Gibsonm, Des Higgins, Julie Thompson, EMBL, Heidelberg, Germany). Phylogenetic analyses were conducted in MEGA6 (
https://www.megasoftware.net) based on the aligned sequences, and phylogenetic trees were constructed using the maximum likelihood method [
5].
Antimicrobial susceptibility was determined at the Korean Institute of Tuberculosis using the CLSI agar microdilution method [
5]. The isolate was susceptible to clarithromycin (minimal inhibitory concentration [MIC]≤0.5 mg/L), but resistant to amikacin (MIC>128 mg/L), cefoxitin (MIC=8 mg/L), ciprofloxacin (MIC>16 mg/L), doxycycline (MIC=8 mg/mL), ethambutol (MIC=4 mg/L), imipenem (MIC>64 mg/L), linezolid (MIC=8 mg/L), moxifloxacin (MIC>16 mg/L), rifampin (MIC=4 mg/L), trimethoprim/sulfamethoxazole (TMP/SMX; MIC=16/304 mg/L), and tobramycin (MIC>32 mg/L). The patient was treated with clarithromycin for three months, and the infection healed.
There is no standard treatment for
M. virginiense infection. Some MTC isolates are susceptible to ethionamide, azithromycin, amikacin, ciprofloxacin, streptomycin, linezolid, cycloserine, TMP/SMX, and some aminoglycosides [
16]. The
M. virginiense isolate described in 2016 was susceptible to clarithromycin, ethambutol, rifabutin, and TMP/SMX, but was resistant to amikacin, rifampin, quinolone, doxycycline, and linezolid [
2]. The pulmonary isolate from Korea was resistant to all antibiotics except clarithromycin [
3]. Because of the limited number of reports, it is not possible to determine whether Korean
M. virginiense strains have higher drug resistance than strains from other countries. However,
M. virginiense infections in Korea should be regarded as possibly multidrug resistant.
According to the American Thoracic Society guidelines, prolonged combination antibiotic therapy with surgical debridement is important, and probably essential, for recovery from NTM infection [
7]. In our case, the choice of antimicrobial therapy was limited as the strain was multidrug-resistant; however, the patient improved clinically following surgery. In addition, as NTM-caused skin and soft tissue infections have been reported following intramuscular injection, cosmetic surgery, and acupuncture, physicians should take this medical history into consideration [
8].
Nonspecific clinical manifestations, lack of knowledge, and inadequate laboratory services often delay diagnosis of NTM infection. Further testing of the unidentified NTM and the introduction of new methods to elucidate various NTM subspecies are necessary to improve the diagnosis and treatment of M. virginiense and other NTM infections. In addition, we recommend performing a pathological examination in cases of prolonged infectious disease, because the causative microorganism may not have been identified. Physicians need to be alert to the possibility of M. virginiense infection because it is not identified by the tests used at the Korean Institute of Tuberculosis. In addition, in cases of NTM infection, it is important to identify the strain correctly and to determine antimicrobial susceptibility.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download