Dear Editor,
Antimicrobial resistance (AMR) is becoming increasingly problematic in Korea [
1], requiring urgent multi-sectional control measures. The massive use of antibiotics could further accelerate the spread of antibiotic-resistant bacteria among humans and animals [
2]. However, the contribution to the human carriage burden from animal reservoirs has not been fully elucidated. Recently, there have been attempts to apply “one health” approach, which is a broad and flexible concept with diverse facets such as establishment of a drug-resistant superbacteria network of the environment, community, and clinical settings [
3]. We investigated the molecular prevalence of cephalosporin-resistant bacteria among livestock, farm environments, and farm workers in Korea, in collaboration with the Korean Centers for Disease Control and Prevention. During the study, we collected four
blaCTX-M-55-carrying
Escherichia coli isolates that were highly suspected to be disseminated in one pig farm in Jeollanam-do Province, Korea, at the same time (August 10, 2017). The purpose of this study was to elucidate the direct spread from different reservoirs (human-animal-environment), verifying the genetic relatedness at the whole-genome sequence level. This study was approved by the Institutional Review Board of National Health Insurance Service Ilsan Hospital, Goyang, Korea (NHIMC 2017-07-041). We obtained informed consent from farm workers.
The four
E. coli isolates were collected from a farm worker (PJFH115, from stool), two pigs (PJFA1104 from stool and PJFA173 from an anal swab), and the pigpen fence (PJFE101) on 10, August, 2017. The strains were stored in skimmed milk at −70℃ until further use. The culture and antimicrobial susceptibility were performed at Yonsei University College of Medicine. DNA of freshly subcultured isolate was extracted using MG Genomic DNA purification kit (MGmed, Seoul, Korea), and 8 µg of input genomic DNA was used. The entire genomes of the four CTX-M-55-producing
E. coli isolates were sequenced using the PacBio RS II (Pacific Biosciences, Menlo Park, CA, USA) platform, and sequences were assembled by Pac Denovo with single-molecule real-time analysis software (ver. 2.3) and annotated by Prokka (ver. 1.12b), provided by Macrogen (Seoul, Korea). The subsequent steps were based on the PacBio Sample Net-Shared Protocol, which is available at
http://pacificbiosciences.com/.
In silico multilocus sequence typing (MLST) [
4], plasmid typing [
5], and searching for acquired resistance genes [
6] were further conducted as previously reported.
The
E. coli isolates PJFH115 belonged to sequence type (ST) 69, PJFA1104 and PJFE101 belonged to the same ST457, whereas PJFA173 was of ST5899. The phylogeny of the
E. coli MLST profile indicated that these three STs were distinct, belonging to separate clades (
Fig. 1A). The chromosomes and plasmids of these isolates contained many genes related to AMR (
Table 1). In particular, the
E. coli isolates from pigs (PJFA1104 and PJFA173) had many AMR genes for aminoglycoside, macrolide, sulfonamide, and phenicol resistance.
All four plasmids encoding CTX-M-55 shared an IncF backbone, including the IncFIB and IncFIC-IncFIB types, with conjugal elements, and those of pig origin had a modified component due to insertion of DNA fragments (
Fig. 1B). The only IncFIB plasmid in the human isolate PJFA115 shared a limited portion with the others, and included only four drug resistance genes, the lowest number overall. A transposon Tn
Ec1 disrupted the open reading frame of
traB in the plasmid of PJFA173. The IncFIC-IncFIB plasmid of pig origin in PJFA173 had a more compact structure compared with those of the other two IncFIC-IncFIB plasmids (PJFE101 and PJFA1104), in terms of the size and the resistance determinants. One composite transposon comprising
dfrA,
aadA1, and
floR interrupted the
traI gene in the plasmid of PJFA1104. The plasmid in PJFA1104 was largely indistinguishable from that in PJFE101, with two exceptions of the truncated
traI and insertion of three copies of IS
15DIV, along with one copy of IS
1 and Tn
2 each downstream from
aadA. Additional plasmids were identified, including an IncI1-type (PJFE101) and IncR-type (PJFA1104) plasmids, which may have been separately acquired during evolution. The position of the
blaCTX-M-55 gene indicated a common ancestor, with IS
Ecp1 upstream and ORF477 following Tn
2 downstream (
Fig. 1C). Continuous evolution, likely involving IS
15DI, is considered to account for the distinct pattern in the plasmid of the human-origin PJFH115 isolate, in which IS
Ecp1 was disrupted by IS
15DI upstream from
blaCTX-M-55, and a complete Tn
2 sequence was identified downstream of the gene. A composite transposition unit was identified in the three IncFIC-IncFIB plasmids.
Our results suggest possible dissemination of an E. coli ST clone between one pig and the farm environment. However, the blaCTX-M-55 plasmid in E. coli PJFH115 from a human specimen was distinct, indicating the lack of transmission between the human and pig or environment. Closely related plasmids from the National Center for Biotechnology Information database (accessed August 21, 2019) were also dissimilar from each other. The closest match was a 96,497-bp plasmid in E. coli ST34 recovered from a swine rectal swab in Thailand in 2016, with 96% coverage and 99.98% nucleic acid identity (GenBank accession, CP041111). The most similar sequence to the other blaCTX-M-55 plasmids was an mcr-1 gene-harboring plasmid in E. coli ST457 recovered from a human rectal swab in the USA in 2016 with 92% coverage and 99.99% nucleic acid identity (GenBank accession, CP029748).
Dorado-García A, et al. [
7] tried to demonstrate an epidemiological linkage of ESBL/AmpC genes and plasmid replicon types between livestock farms and the general population using pooled data, recovered from 35 studies in the Netherlands comprising more than 27,000 samples. In that study, isolates from people in the general population had higher similarities to those from human clinical settings, surface and sewage water, and wild birds, while similarities to livestock or food reservoirs were lower. Compared to that study, we evaluated direct transfer to occupational persons and also failed to find the evidence of the transmission from pig or environment to human. However, the finding of many plasmid-mediated resistance genes in healthy pigs highlights the need to curb antibiotic use in food-producing animals to prevent the generation of resistance pool and spread of antibiotic-resistant bacteria in animals, contamination of surrounding environments, and changing environment microbiome [
89]. Prudent use of antibiotics and continued surveillance of resistant organisms in human and animal sectors are essential.
Figures and Tables
Fig. 1
Genetic relationships and plasmid structures of the four CTX-M-55-producing Esherichia coli isolates. (A) Phylogeny of the E. coli sequence types (STs). The phylogeny was constructed with E. coli MLST profiles (Phyloviz, http://online.phyloviz.net/index). The nodes indicating the three STs of interest are set out in colored boxes and magnified. (B, C) Features of four blaCTX-M-55 harboring plasmids in E. coli.
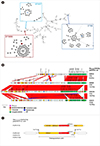
Table 1
Genomic features of the CTX-M-55-producing E. coli isolates recovered from the three interfaces at a pig farm

|
E. coli strain |
Isolated from |
MLST (adk-fumC-gyrB-icd-mdh-purA-recA) |
Chromosome |
Plasmid (bp) |
|
Size (bp) |
Resistance gene |
Incompatibility type (bp) |
Resistance gene |
|
PJFH115 |
Farm worker |
ST69 (21-35-27-6-5-5-4) |
5,001,319 |
sul2, aph(3’’)-Ib, aph(6)-Id, tetA, floR
|
IncFIB (88,456) |
blaCTX-M-55, aac(3)-IId, catA, qnrS
|
|
PJFA1104 |
Pig |
ST457 (101-88-97-108-26-79-2) |
5,149,137 |
Not identified |
IncFIC-IncFIB (149,674) |
blaCTX-M-55, blaTEM-1B, aadA22, aadA1, aph(3’’)-Ib, aph(3’)-Ia, aph(6)-Id, lnu(F),
|
|
sul2, sul3, catA2, floR, tet(A), dfrA14
|
|
IncR (120,439) |
blaTEM-1B
|
|
PJFA173 |
Pig |
ST5899 (20-45-41-43-5-32-413) |
5,001,319 |
aph(3’’)-Ib, aph(6)-Id, aph(3’)-Ia, tet(B), sul2 |
IncFIC-IncFIB (132,765) |
blaCTX-M-55, aadA22, aadA1, aac(3)-IIa, lnu(F), sul3, floR
|
|
IncX1 (117,928) |
Not identified |
|
PJFE101 |
Pigpen fence |
ST457 (101-88-97-108-26-79-2) |
4,919250 |
Not identified |
IncFIC-IncFIB (132,610) |
blaCTX-M-55, blaTEM-1B, aadA22, aph(3’’)-Ib, aph(3’)-Ia, aph(6)-Id, lnu(F), sul2, catA2, tetA, dfrA14
|
|
IncI1 (114,979) |
aadA1, aph(3’)-Ia, ant(2’’)-Ia, sul3
|






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download