This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Triplication of the long arm of chromosome 1 is a rare chromosomal abnormality reported in hematologic malignancies such as acute myeloid leukemia (AML), acute lymphoblastic leukemia, myelodysplastic syndrome (MDS), lymphoma, myeloproliferative neoplasm (MPN), multiple myeloma, and Fanconi anemia (FA) [
12]. Among these, only one case of primary myelofibrosis (PMF) has revealed 1q triplication in bone marrow karyotyping. Furthermore, 1q triplication as a sole cytogenetic abnormality is extremely rare, with only four cases identified in patients with AML, MDS, FA, or PMF [
123]. We report the first case of solitary 1q triplication in a patient with PMF without other hematologic malignancies. This study was approved by the Institutional Review Board of Hanyang University Medical Center, Seoul, Korea (HYUH 2019-09-009).
A 38-year-old female patient was admitted to the pulmonology department of Hanyang University Seoul hospital, Seoul, Korea, with chronic cough and was diagnosed as having miliary tuberculosis in May 2017. Bicytopenia was found at admission, and she was transferred to the hemato-oncology department for further evaluation. Informed consent was obtained from the patient before bone marrow study and genetic testing.
Her complete blood count (CBC) revealed a white blood cell count of 1.7×10
9/L (segmented neutrophils, 50%; band form neutrophils, 2%; lymphocytes, 44%; monocytes, 3%; and eosinophils, 1%), an Hb level of 62 g/L, and a platelet count of 272×10
9/L. The initial mean corpuscular volume was decreased to 65.8 fL. A peripheral blood smear showed poikilocytosis with teardrop-shaped red blood cells (
Fig. 1A). An abdominal computed tomography showed mild hepatosplenomegaly, and serum lactate dehydrogenase level was elevated to 5.3 µkat/L (reference range: 2.3–4.5 µkat/L).
The initial bone marrow biopsy done in May 2017 revealed inadequate aspirate due to dry tap. The core biopsy showed normal cellularity (50%) with megakaryocytic hyperplasia and atypia. The megakaryocytes were increased in number and formed small to large clusters with anisocytosis. Osteosclerosis was not significant (
Fig. 1B). Grade MF-2 bone marrow fibrosis was identified by reticulin stain (
Fig. 1C), and grade 1 collagen deposition was detected by Masson's trichrome stain. Neither necrosis nor granuloma was observed in the bone marrow. Chromosome testing showed solitary trp(1)(q21q32) in all 20 metaphase cells (
Fig. 1D).
JAK2-V617F,
CALR, and
MPL mutations were all tested negative. In addition, no other pathogenic variant was identified in the AML gene panel testing including 45 genes such as
ASXL1,
EZH2,
TET2,
IDH1,
IDH2,
SRSF2, and
SF3B1. Acid-fast bacilli staining and tuberculosis culture of bone marrow samples were negative. The patient was diagnosed as having PMF based on the 2016 revision of the WHO classification [
4].
Following anti-tuberculosis therapy with oral isoniazid, rifampicin, ethambutol, and pyridoxine, the miliary tuberculosis improved. However, the bicytopenia persisted for eight months after treatment; the follow-up (21 months after the first evaluation) bone marrow study and chromosome test results were the same as the previous ones.
Of the previous two cases of 1q triplication in MPN patients, one was reported during the follow-up of chronic myeloid leukemia (CML), and the other case was detected as the sole cytogenetic abnormality in a PMF patient with a
BCR/ABL1 negative state following CML treatment [
2]; in this case, unlike the previous reports, 1q triplication was reported in a PMF patient without evidence of other hematologic malignancies (
Table 1).
Approximately 12% of patients with PMF have been reported as triple-negative for
JAK2-V617F,
CALR, and
MPL mutations [
4]. In these cases, searching for frequent accompanying mutations is helpful in determining the clonality of the disease [
45]. In our case, no mutation was found in the AML gene panel test; thus, the only evidence of clonality was 1q triplication.
As previously established, 1q duplication is one of the most common abnormalities in PMF [
567]. In addition, 33% of the reported 1q triplication cases (11/33) and 60% of the solitary 1q triplication cases (3/5), including the present study, share a triplicated region on 1q21 to 1q32 [
123]. Thus, there might be a correlation between the 1q21 to 1q32 region and the pathogenesis of hematologic malignancies.
In conclusion, this is the first case of sole1q triplication in a patient with PMF without other hematologic malignancies. Further study is needed to confirm the pathogenesis of 1q triplication in the myeloproliferative process.
Figures and Tables
Fig. 1
Peripheral blood, bone marrow, and karyotypic findings of the patient. (A) Peripheral blood smear showing teardrop-shaped cells (arrow) and anisocytosis (Wright-Giemsa stain, ×400). (B) Bone marrow biopsy demonstrating proliferative and clustered megakaryocytes with atypia (arrow) (H&E stain, ×200). (C) Grade MF-2 reticulin fibrosis (arrow) (reticulin stain, ×400). (D) Karyotyping showing 46,XX,trp(1)(q21q32) (arrow).
Abbreviations: H&E, Hematoxylin and Eosin; MF, myelofibrosis.
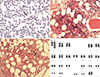
Table 1
Summary of 1q triplication in patients with MPN

|
Patient, age (yr)/sex |
Diagnosis |
Triplication region |
Additional chromosomal abnormalities |
Presence of another clonal marker |
Reference |
|
38/F |
PMF |
trp(1)(q21q32) in all 20 metaphase cells |
None |
None (by AML gene panel test) |
Present study |
|
28/M |
CML (follow-up period) |
trp(1)(q24q41) in all 20 metaphase cells |
t(9;22)(q34;q11.2) in all 20 metaphase cells analyzed |
NA |
Park, et al. [2] |
|
57/F |
CML → PMF |
trp(1)(q21q32) in 6/22 metaphase cells |
None |
NA |
Park, et al. [2] |






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download