2. Scott DL. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther. 2012; 91:30–43. PMID:
22166850.
4. Isaacs J, Gonçalves J, Strohal R, Castañeda-Hernández G, Azevedo V, Dörner T, et al. The biosimilar approval process: how different is it? Considerations Med. 2017; 1:3–6.
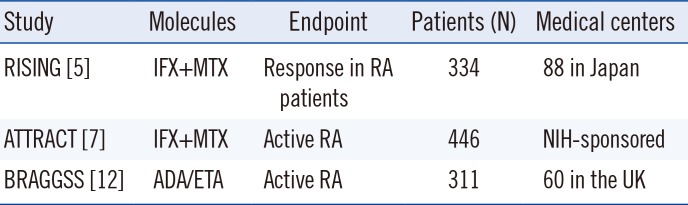
5. Takeuchi T, Miyasaka N, Inoue K, Abe T, Koike T. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol. 2009; 19:478–487. PMID:
19626391.
6. Svenson M, Geborek P, Saxne T, Bendtzen K. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology (Oxford). 2007; 46:1828–1834. PMID:
18032541.
7. St. Clair E, Wagner C, Fasanmade A, Wang B, Schaible T, Kavanaugh A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002; 46:1451–1459. PMID:
12115174.
8. Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. 2013; 72:1947–1955. PMID:
23223420.
9. Maneiro JR, Salgado E, Gomez-Reino JJ. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated inflammatory conditions: systematic review and meta-analysis. JAMA Intern Med. 2013; 173:1416–1428. PMID:
23797343.
10. Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014; 73:492–509. PMID:
24161836.
11. Hart MH, de Vrieze H, Wouters D, Wolbink GJ, Killestein J, de Groot ER, et al. Differential effect of drug interference in immunogenicity assays. J Immunol Methods. 2011; 372:196–203. PMID:
21824477.
12. Jani M, Chinoy H, Warren R, Griffiths C, Plant D, Fu B, et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol. 2015; 67:2011–2019. PMID:
26109489.
13. Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013; 72:165–178. PMID:
23178294.
14. Smith HW, Butterfield A, Sun D. Detection of antibodies against therapeutic proteins in the presence of residual therapeutic protein using a solid-phase extraction with acid dissociation (SPEAD) sample treatment prior to ELISA. Regul Toxicol Pharmacol. 2007; 49:230–237. PMID:
17869396.
15. Llinares-Tello , Rosas-Gómez de Salazar J, Senabre-Gallego JM, Santos-Soler G, Santos-Ramírez C, Salas-Heredia E, et al. Practical application of acid dissociation in monitoring patients treated with adalimumab. Rheumatol Int. 2014; 34:1701–1708. PMID:
24816715.
16. Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009; 68:1739–1745. PMID:
19019895.
17. Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006; 54:3782–3789. PMID:
17133559.
18. Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011; 305:1460–1468. PMID:
21486979.
19. Dore RK, Mathews S, Schechtman J, Surbeck W, Mandel D, Patel A, et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly inpatients with rheumatoid arthritis. Clin Exp Rheumatol. 2007; 25:40–46. PMID:
17417989.
20. de Vries MK, van der Horst-Bruinsma IE, Nurmohamed MT, Aarden LA, Stapel SO, Peters MJ, et al. Immunogenicity does not influence treatment with etanercept in patients with ankylosing spondylitis. Ann Rheum Dis. 2009; 68:531–535. PMID:
18375542.
21. Moots RJ, Xavier RM, Mok CC, Rahman MU, Tsai WC, Al-Maini MH, et al. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real-world clinical practice, non-interventional study. PLoS One. 2017; 12:e0175207. PMID:
28448562.
22. Conti F, Ceccarelli F, Massaro L, Cipriano E, Di Franco M, Alessandri C, et al. Biological therapies in rheumatic diseases. Clin Ter. 2013; 164:e413–e428. PMID:
24217844.
23. Schaeverbeke T, Truchetet ME, Kostine M, Barnetche T, Bannwarth B, Richez C. Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology (Oxford). 2016; 55:210–220. PMID:
26268816.
24. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008; 117:244–279. PMID:
18155297.
25. Matucci A, Cammelli D, Cantini F, Goletti D, Marino V, Milano GM, et al. Influence of anti-TNF immunogenicity on safety in rheumatic disease: a narrative review. Expert Opin Drug Saf. 2016; 15:3–10. PMID:
27924646.
26. Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017; 31:299–316. PMID:
28612180.
27. Quistrebert J, Hässler S, Bachelet D, Mbogning C, Musters A, Tak PP, et al. Incidence and risk factors for adalimumab and infliximab anti-drug antibodies in rheumatoid arthritis: a European retrospective multicohort analysis. Semin Arthritis Rheum. 2019; 48:967–975. PMID:
30420245.
28. Magill L, Adriani M, Berthou V, Chen K, Gleizes A, Hacein-Bey-Abina S, et al. Low percentage of signal regulatory protein α/β+ memory B cells in blood predicts development of anti-drug antibodies (ADA) in adalimumab-treated rheumatoid arthritis patients. Front Immunol. 2018; 9:2865. PMID:
30568660.
29. Bartelds S, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumor necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010; 69:817–821. PMID:
19581278.
30. Chen DY, Chen YM, Tsai WC, Tseng JC, Chen YH, Hsieh CW, et al. Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis. 2015; 74:e16. PMID:
24442879.
31. Jani M, Chinoy H, Warren RB, Fu B, Griffiths CE, Morgan AW, et al. SAT0052 Influence of immunogencity and drug levels on the efficacy of long-term treatment of rheumatoid arthritis with adalimumab and etanercept: a UK-based prospective study. Ann Rheum Dis. 2014; 73:608.
32. Eng G, Fana V, Omerovic E, Højsgaard P, Lindegaard HM, Jensen EK, et al. Presence of antibodies to adalimumab and infliximab in patients with rheumatoid arthritis in clinical remission. Ann Rheum Dis. 2013; 72:230.
33. Ducourau E, Mulleman D, Paintaud G, Miow Lin DC, Lauféron F, Ternant D, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatoid disease. Arthritis Res Ther. 2011; 13:R105. PMID:
21708018.
34. Siljehult F, Arlestig L, Eriksson C, Rantapää-Dahlqvist S. Concentration of infliximab and anti-drug antibodies in relation to clinical response in patients with rheumatoid arthritis. Scand J Rheumatol. 2018; 47:345–350. PMID:
29701536.
35. Chen DY, Chen YM, Hung WT, Chen HH, Hsieh CW, Chen YH, et al. Immunogenicity, drug trough levels and therapeutic response in patients with rheumatoid arthritis and ankyloses spondyloarthritis after 24-week golimumab treatment. Ann Rheum Dis. 2015; 74:2261–2264. PMID:
26443609.
36. Krieckaert CL, Nurmohamed MT, Wolbink GJ. Methotrexate reduces immunogenicity in adalimumab treated rheumatoid arthritis patients in a dose dependent manner. Ann Rheum Dis. 2012; 71:1914–1915. PMID:
22586169.
37. Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, et al. Comparative immunogenicity of TNF Inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs. 2015; 29:241–258. PMID:
26280210.
38. Wu C, Wang S, Xian P, Yang L, Chen Y, Mo X, et al. Effect of anti-TNF antibodies on clinical response in rheumatoid arthritis patients: a meta-analysis. BioMed Res Int. 2016; 2016:7185708. PMID:
27556040.
39. Krintel SB, Grunert VP, Hetland ML, Johansen JS, Rothfuss M, Palermo G, et al. The frequency of anti-infliximab antibodies in patients with rheumatoid arthritis treated in routine care and the association with adverse drug reaction and treatment failure. Rheumatology (Oxford). 2013; 52:1245–1253. PMID:
23459699.
40. Hoxha A, Calligaro A, Tonello M, Ramonda R, Carletto A, Paolazzi G, et al. The clinical relevance of early anti-adalimumab antibodies detection in rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: a prospective multicentre study. Joint Bone Spine. 2016; 83:167–171. PMID:
26750762.
41. Pascual-Salcedo D, Plasencia C, Ramiro S, Nuño L, Bonilla G, Nagore D, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology. 2011; 50:1445–1452. PMID:
21427177.
42. Genovese MC, Pacheco-Tena C, Covarrubias A, Leon G, Mysler E, Keiserman M, et al. Longterm safety and efficacy of subcutaneous abatacept in patients with rheumatoid arthritis: 5-year results from a phase IIIb trial. J Rheumatol. 2018; 45:1085–1092. PMID:
29657147.
43. Brunner HI, Tzaribachev N, Vega-Cornejo G, Louw I, Berman A, Calvo Penadés I, et al. Paediatric Rheumatology International Trials Organisation (PRINTO) and the Pediatric Rheumatology Collaborative Study Group (PRCSG). Subcutaneous abatacept in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase III open-label study. Arthritis Rheumatol. 2018; 70:1144–1154. PMID:
29481737.
44. Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017; 76:1078–1085. PMID:
28007755.
45. Benucci M, Meacci F, Grossi V, Infantino M, Manfredi M, Bellio E, et al. Correlations between immunogenicity, drug levels, and disease activity in an Italian cohort of rheumatoid arthritis patients treated with tocilizumab. Biologics. 2016; 10:53–58. PMID:
27041992.
46. Sigaux J, Hamze M, Daien C, Morel J, Krzysiek R, Pallardy M, et al. Immunogenicity of tocilizumab in patients with rheumatoid arthritis. Joint Bone Spine. 2017; 84:39–45. PMID:
27369643.
47. Mok CC. Rituximab for the treatment of rheumatoid arthritis: an update. Drug Des Devel Ther. 2013; 8:87–100.
48. Karle A, Spindeldreher S, Kolbinger F. Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity. MAbs. 2016; 8:536–550. PMID:
26817498.
49. Verstockt B, Deleenheer B, Van Assche G, Vermeire S, Ferrante M. A safety assessment of biological therapies targeting the IL-23/IL-17 axis in inflammatory bowel diseases. Expert Opin Drug Saf. 2017; 16:809–821. PMID:
28573876.
50. European Medicines Agency. Guideline on similar biological medicinal products (Revision). CHMP/437/04 Rev 1. London, UK: 2014. p. 1–7.
51. Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013; 72:1605–1612. PMID:
23687259.
52. Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013; 72:1613–1620. PMID:
23687260.
53. Reinisch W, Jahnsen J, Schreiber S, Danese S, Panés J, Balsa A, et al. Evaluation of the cross-reactivity of antidrug antibodies to CT-P13 and infliximab reference product (Remicade): an analysis using immunoassays tagged with both agents. BioDrugs. 2017; 31:223–237. PMID:
28497221.
54. Lamb YN, Scott LJ, Deeks ED. SB2: an infliximab biosimilar. BioDrugs. 2017; 31:461–464. PMID:
28803431.
55. Emery P, Vencovský J, Sylwestrzak A, Leszczyński P, Porawska W, Baranauskaite A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017; 76:51–57. PMID:
26150601.
56. Emery P, Vencovský J, Sylwestrzak A, Leszczyński P, Porawska W, Stasiuk B, et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis. 2017; annrheumdis-2017-211591.
57. European Medicines Agency. European public assessment report (EPAR): Benepali (etanercept) 2016.
http://www.ema.europa.eu.
58. Emery P, Vencovský J, Sylwestrzak A, Leszczynski P, Porawska W, Baranauskaite A, et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology (Oxford). 2017; 56:2093–2101. PMID:
28968793.
59. Poetzl J, Arlt I, von Richter O, Wöhling H, Afonso M, Schaffar G. State-of-the-art immunogenicity evaluation in phase 3 confirmatory study (EGALITY) with etanercept biosimilar GP2015. J Eur Acad Dermatol Venereol. 2018; 32:e130–e132. PMID:
29024096.
60. Yoo DH, Suh CH, Shim SC, Jeka S, Cons-Molina FF, Hrycaj P, et al. A multicentre randomised controlled trial to compare the pharmacokinetics, efficacy and safety of CT-P10 and innovator rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017; 76:566–570. PMID:
27624791.
61. Coiffier B, Sancho JM, Jurczak W, Kim JS, Nagarkar RV, Zhavrid E, et al. Pharmacokinetic and safety of CT-P10, a biosimilar candidate to the rituximab reference product, in patients with newly diagnosed advanced stage follicular lymphoma (AFL). Blood. 2016; 128:1807.
62. Smolen JS, Cohen SB, Tony HP, Scheinberg M, Kivitz A, Balanescu A, et al. A randomised, double-blind trial to demonstrate bioequivalence of GP2013 and reference rituximab combined with methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis. 2017; 76:1598–1602. PMID:
28637670.
63. Garcês S, Antunes M, Benito-Garcia E, da Silva JC, Aarden L, Demengeot J, et al. A preliminary algorithm introducing immunogenicity assessment in the management of patients with RA receiving tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2014; 73:1138–1143. PMID:
23666932.
64. Park W, Yoo DH, Jaworski J, Brzezicki J, Gnylorybov A, Kadinov V, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CTP13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016; 18:25. PMID:
26795209.
65. Yoo DH, Racewicz A, Brzezicki J, Yatsyshyn R, Arteaga ET, Baranauskaite A, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2016; 18:82. PMID:
27038608.
66. Aarden L, Ruuls SR, Wolbink G. Immunogenicity of anti-tumor necrosis factor antibodies toward improved methods of anti-antibody measurement. Curr Opin Immunol. 2008; 20:431–435. PMID:
18619538.
67. Wolbink GJ, Aarden LA, Dijkmans BA. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol. 2009; 21:211–215. PMID:
19399992.
68. Hart MH, de Vrieze H, Wouters D, Wolbink GJ, Killestein J, de Groot ER, et al. Differential effect of drug interference in immunogenicity assays. J Immunol Methods. 2011; 372:196–203. PMID:
21824477.
69. Salimi-Moosavi H, Rathanaswami P, Rajendran S, Toupikov M, Hill J. Rapid affinity measurement of protein-protein interactions in a microfluidic platform. Anal Biochem. 2012; 426:134–141. PMID:
22542978.
70. Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins. EMEA/CHMP/BMWP/14327/2006. p. 7–9.
71. Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. EMA/CHMP/BMWP/86289/2010 (Updated 2010). p. 9–12.
72. Guidi L, Pugliese D, Panici Tonucci T, Berrino A, Tolusso B, Basile M, et al. Therapeutic drug monitoring is more cost-effective than a clinically-based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multicentre study. J Crohns Colitis. 2018; 12:1079–1088.
73. Bader L, Solberg SM, Kaada SH, Bolstad N, Warren DJ, Gavasso S, et al. Assays for infliximab drug levels and antibodies: a matter of scales and categories. Scand J Immunol. 2017; 86:165–170. PMID:
28561325.
74. Steenholdt C, Ainsworth MA, Tovey M, Klausen TW, Thomsen OO, Brynskov J, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther Drug Monit. 2013; 35:530–538. PMID:
23765033.
75. Steenholdt C, Bendtzen K, Brynskov J, Thomsen OO, Ainsworth MA. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn's disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol. 2014; 109:1055–1064. PMID:
24796769.
76. Vande Casteele N, Buurman DJ, Sturkenboom MGG, Kleibeuker JH, Vermeire S, Rispens T, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012; 36:765–771. PMID:
22928581.
77. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015; 12:205–217. PMID:
25732745.
78. Enciso IP, Paredes LF, Sanchez-Ramon S, Mendoza JL, Cristina Alba, Olivares D, et al. Comparison of four assay kits for measuring infliximab trough levels and antibodies to infliximab in patients with inflammatory bowel disease. Gastroenterology. 2016; 4:S415.
79. Guiotto C, Daperno M, Frigerio F, Vizzini M, Cerruti R, Ercole E, et al. Clinical relevance and inter-test reliability of anti-infliximab antibodies and infliximab trough levels in patients with inflammatory bowel disease. Dig Liver Dis. 2016; 48:138–143. PMID:
26614644.
80. Lee MW, Connor S, Ng W, Toong CM. Comparison of infliximab drug measurement across three commercially available ELISA kits. Pathology. 2016; 48:608–612. PMID:
27567230.
81. Malickova K, Duricova D, Bortlik M, Hind’oš M, Machková N, Hrubá V, et al. Serum trough infliximab levels: a comparison of three different immunoassays for the monitoring of CT-P13 (infliximab) treatment in patients with inflammatory bowel disease. Biologicals. 2016; 44:33–36. PMID:
26603635.
82. Schmitz EM, van de Kerkhof D, Hamann D, van Dongen JL, Kuijper PH, Brunsveld L, et al. Therapeutic drug monitoring of infliximab: performance evaluation of three commercial ELISA kits. Clin Chem Lab Med. 2016; 54:1211–1219. PMID:
26587745.
83. Van Bezooijen JS, Koch BC, Doorn MV, Prens EP, Gelder TV, Schreurs MW. A comparison of three assays to quantify infliximab, adalimumab and etanercept serum concentrations. Ther Drug Monit. 2016; 38:432–438. PMID:
27120178.
84. Freeman K, Taylor-Phillips S, Connock M, Court R, Tsertsvadze A, Shyangdan D, et al. Test accuracy of drug and antibody assays for predicting response to antitumor necrosis factor treatment in Crohn's disease: a systematic review and meta-analysis. BMJ Open. 2017; 7:e014581.
85. Pérez I, Fernández L, Sánchez-Ramon S, Alba C, Zatarain A, Cañas M, et al. Reliability evaluation of four different assays for therapeutic drug monitoring of infliximab levels. Therap Adv Gastroenterol. 2018; 11:1756284818783613.
86. Afonso J, de Sousa HT, Rosa I, Carvalho J, Dias CC, Magro F. Therapeutic drug monitoring of CT-P13: a comparison of four different immunoassays. Therap Adv Gastroenterol. 2017; 10:661–671.
87. Schmitz EMH, Benoy-De Keuster S, Meier AJL, Scharnhorst V, Traksel RAM, Broeren MAC, et al. Therapeutic drug monitoring (TDM) as a tool in the switch from infliximab innovator to biosimilar in rheumatic patients: results of a 12-month observational prospective cohort study. Clin Rheumatol. 2017; 36:2129–2134. PMID:
28593609.
88. Afonso J, Lopes S, Goncalves R, Caldeira P, Lago P, Tavares de Sousa H, et al. Proactive therapeutic drug monitoring of infliximab: a comparative study of a new point-of-care quantitative test with two established ELISA assays. Aliment Pharmacol Ther. 2016; 44:684–692. PMID:
27507790.
89. Magro F, Afonso J, Lopes S, Coelho R, Gonçalves R, Caldeira P, et al. Clinical performance of an infliximab rapid quantification assay. Therap Adv Gastroenterol. 2017; 10:651–660.
90. Nasser Y, Labetoulle R, Harzallah I, Berger AE, Roblin X, Paul S, et al. Comparison of point of care and classical immunoassay for the monitoring Infliximab and antibodies against Infliximab in IBD. Dig Dis Sci. 2018; 63:2714–2721. PMID:
29948562.
91. Meacci F, Manfredi M, Infantino M, Grossi V, Benucci M. Anti Etanercept and anti SB4 antibodies detection: impact of the assay method. Ann Rheum Dis. 2016; 75:e39. PMID:
27130906.
92. Meacci F, Manfredi M, Infantino M, Grossi V, Benucci M. Infliximab and CT-P13 immunogenicity assessment in PLANETAS and PLANETRAS main and extension studies: utility of laboratory methods description. Ann Rheum Dis. 2016; 75:e62. PMID:
27401744.
93. Qin X, Rui J, Xia Y, Mu H, Song SH, Raja Aziddin RE, et al. Multi-center performance evaluations of Tacrolimus and Cyclosporine electrochemiluminescence immunoassays in the Asia-Pacific region. Ann Lab Med. 2018; 38:85–94. PMID:
29214751.
94. Choi R, Jeong BH, Koh WJ, Lee SY. Recommendations for optimizing tuberculosis treatment: therapeutic drug monitoring, pharmacogenetics, and nutritional status considerations. Ann Lab Med. 2017; 37:97–107. PMID:
28028995.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download