INTRODUCTION
MATERIALS and METHODS
HGK cultures
Immunoblotting
Antibody microarray analysis of MMPs
Zymogram analysis
Immunocytochemistry and measurement of ECJs
Immunohistochemistry of vitamin D receptor (VDR)
Statistical analysis
RESULTS
 | Figure 1TNF-α suppresses the development of ECJs in HGKs in vitro. Cells were cultured for 24 hours for the establishment of the ECJs and then treated with various amounts of TNF-α (10, 40, 100 ng/mL). (A) ECJ development was detected by immunocytochemical staining of E-cadherin expression (red). In addition, F-actin (green) was stained with FITC-phalloidin and nuclei were stained with DAPI (blue). The lower right pictures in each set of pictures are the merged images of the E-cadherin, F-actin, and nuclei images.(B) The average ratio of the development of ECJs per cell was calculated.TNF-α: tumor necrosis factor-alpha, ECJ: E-cadherin junction, HGK: human gingival keratinocyte, DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride.
a)P<0.05 versus b)Nonparametric Kruskal-Wallis test and post hoc multiple comparison with the Dunn test.
|
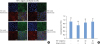 | Figure 2Vitamin D suppresses the dissociation of the ECJs induced by TNF-α in HGKs in vitro. Cells were cultured for 24 hours for the establishment of ECJs and pretreated with vitamin D (10, 100 nM) or without it for an additional 24 hours. Then, cells were cultured with or without TNF-α for 24 hours along with vitamin D. (A) ECJ development was detected by immunocytochemical staining of E-cadherin expression (red). In addition, F-actin (green) was stained with FITC-phalloidin and nuclei were stained with DAPI (blue). The lower right pictures in each set of pictures are the merged images of the E-cadherin, F-actin, and nuclei images. (B) The average ratio of the development of ECJs per cell was calculated.ECJ: E-cadherin junction, TNF-α: tumor necrosis factor-alpha, HGK: human gingival keratinocyte, DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride.
a)P<0.05 versus b)Nonparametric Kruskal-Wallis test and post hoc multiple comparison with the Dunn test.
|
Disruption of ECJs by MMP-9
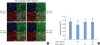 | Figure 3TIMP-1 suppresses the dissociation of ECJs induced by TNF-α in HGKs in vitro. Cells were cultured for 24 hours for the establishment of the ECJs and then treated with TNF-α (40 ng/mL), with or without TIMP-1 (20 ng/mL) for an additional 24 hours. (A) ECJ development was followed by immunocytochemical staining of E-cadherin expression (red). In addition, F-actin (green) was stained with FITC-phalloidin and nuclei were stained with DAPI (blue). The lower right pictures in each set of pictures are the merged images of the E-cadherin, F-actin, and nuclei images. (B) The average ratio of the development of ECJs per cell was calculated by taking the mean of the values that were obtained by dividing the length of the E-cadherin expressed at cell junctions by the total perimeter of each cell.TIMP-1: tissue inhibitor of metalloproteinase-1, ECJ: E-cadherin junction, TNF-α: tumor necrosis factor-alpha, HGK: human gingival keratinocyte, DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride.
a)P<0.05 versus b)Nonparametric Kruskal-Wallis test and post hoc multiple comparison with the Dunn test.
|
 | Figure 4TNF-α upregulates the secretion of MMP-9, as shown by antibody microarray analysis, and gelatinolytic digestion by the secreted MMP-9, as demonstrated by gelatin zymography in HGKs in vitro, respectively. (A) Cells were cultured for 24 hours for the establishment of the ECJs and then treated with TNF-α (40 ng/mL). Antibody microarray analysis of MMPs was performed by applying conditioned culture medium to the antibody microarray chip for MMPs. The table contains a list of the MMPs or TIMPs in the array. The numbers under the MMP-9 blots indicate the intensity of MMP-9 standardized using the positive controls as references (boxes). (B) Cells were cultured for 24 hours for the establishment of the ECJs and then treated with various amounts of TNF-α (10, 40, 100 ng/mL). Secretion of MMP-9 into the culture medium was assessed by gelatin zymogram analysis. The numbers under the MMP-9 bands indicate the activity of MMP-9.TNF-α: tumor necrosis factor-alpha, MMP-9: matrix metalloproteinase-9, HGK: human gingival keratinocyte, ECJ: E-cadherin junction, Pos: positive control for the chip reactions, Neg: negative control for the chip reactions.
|
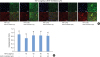 | Figure 5A specific inhibitor of MMP-9 reverses the effect of TNF-α on the development of ECJs in HGKs in vitro. Cells were cultured for 24 hours for the establishment of the ECJs and then treated with TNF-α (40 ng/mL) along with various amounts of an MMP-9 inhibitor (5, 20, or 50 µM) or without it for an additional 24 hours. (A) ECJ development was followed by immunocytochemical staining of E-cadherin expression (red). In addition, F-actin (green) was stained with FITC-phalloidin and nuclei were stained with DAPI (blue). The lower right pictures in each set of pictures are the merged images of the E-cadherin, F-actin, and nuclei images. (B) The average extent of the development of ECJs per cell was calculated by taking the mean of the values that were obtained by dividing the length of the E-cadherin expressed at cell junctions by the total perimeter of each cell.MMP-9: matrix metalloproteinase-9, TNF-α: tumor necrosis factor-alpha, ECJ: E-cadherin junction, HGK: human gingival keratinocyte, DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride.
a)P<0.05 versus b)Nonparametric Kruskal-Wallis test and post hoc multiple comparison with the Dunn test.
|
 | Figure 6MMP-9 induces the dissociation of ECJs in HGKs in vitro. Cells were cultured for 24 hours for the establishment of the ECJs and then treated with various amounts of MMP-9 (10, 50, 100 ng/mL). (A) ECJ development was followed by immunocytochemical staining of E-cadherin expression (red). In addition, F-actin (green) was stained with FITC-phalloidin and nuclei were stained with DAPI (blue). The lower right pictures in each set of pictures are the merged images of the E-cadherin, F-actin, and nuclei images. (B) The average extent of the development of ECJs per cell was calculated by taking the mean of the values that were obtained by dividing the length of the E-cadherin expressed at cell junctions by the total perimeter of each cell.MMP-9: matrix metalloproteinase-9, ECJ: E-cadherin junction, HGK: human gingival keratinocyte, DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride.
a)P<0.05 versus b)Nonparametric Kruskal-Wallis test and post hoc multiple comparison with the Dunn test.
|
Reinforcement of ECJs by vitamin D
 | Figure 7Vitamin D downregulates the TNF-α-induced secretion of MMP-9 and the gelatinolytic digestion by the MMP-9 upregulated by TNF-α in HGKs in vitro. Cells were cultured for 24 hours for the establishment of ECJs and pretreated with or without vitamin D (10 or 100 nM) for an additional 24 hours. Then, cells were cultured with or without TNF-α (40 ng/mL) along with vitamin D for 24 and 48 hours (A) or 48 hours (B). (A) Antibody microarray analysis of MMPs was performed by applying conditioned culture medium to the antibody microarray of MMPs. Details on the arrays are shown in the legend of Figure 4A. The numbers under the MMP-9 blots indicate the intensity of MMP-9 standardized using the positive controls as references (boxes). (B) Secretion of MMP-9 into the culture medium was assessed by gelatin zymogram analysis. The numbers under the MMP-9 bands indicate the activity of MMP-9.TNF-α: tumor necrosis factor-alpha, MMP-9: matrix metalloproteinase-9, HGK: human gingival keratinocyte, ECJ: E-cadherin junction.
|
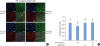 | Figure 8NF-κB inhibition by Bay 11-7082 reverses the TNF-α-induced effects on HGKs in vitro. Cells were cultured for 24 hours for the establishment of ECJs and then treated with TNF-α (40 ng/mL) along with Bay 11-7082 (Bay11; 10 nM) or without it for an additional 24 hours. (A) ECJ development was followed by immunocytochemical staining of E-cadherin expression (red). In addition, F-actin (green) was stained with FITC-phalloidin and nuclei were stained with DAPI (blue). The lower right pictures in each set of pictures are the merged images of the E-cadherin, F-actin, and nuclei images. (B) The average ratio of the development of ECJs per cell was calculated by taking the mean of the values which were obtained by dividing the length of the E-cadherin expressed at cell junctions by the total perimeter of each cell.NF-κB: nuclear factor kappa B, TNF-α: tumor necrosis factor-alpha, HGK: human gingival keratinocyte, ECJ: E-cadherin junction, DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride.
a)P<0.05 versus b)Nonparametric Kruskal-Wallis test and post hoc multiple comparison with the Dunn test.
|
 | Figure 9The inhibition of NF-κB signaling by Bay 11-7082 lowers the secretion of MMP-9 into the culture medium and the gelatinolytic digestion by the secreted MMP-9, upregulated by TNF-α. Cells were cultured for 24 hours for the establishment of ECJs and then treated with TNF-α (40 ng/mL) along with Bay 11-7082 (Bay11; 10 nM) or without it for an additional 24 hours. (A) Antibody microarray analyses of MMPs were performed by applying culture medium to the MMPs antibody microarray chip. Representative data from the triplicate measurements are shown. Details on the arrays are shown in the legend of Figure 4A. The numbers under the MMP-9 blots indicate the intensity of MMP-9 standardized using the positive controls as references (boxes). (B) Secretion of MMP-9 into the culture medium was assessed by gelatin zymogram analysis. The numbers under the MMP-9 bands indicate the activity of MMP-9.NF-κB: nuclear factor kappa B, MMP-9: matrix metalloproteinase-9, TNF-α: tumor necrosis factor-alpha, ECJ: E-cadherin junction.
|
 | Figure 10Vitamin D inhibits TNF-α-induced NF-κB signaling, as shown by western blotting. Cells were cultured for 48 hours and then pretreated with vitamin D (10, 100, or 1,000 nM) or without it for 24 hours. Before cell lysis for western blotting, cells were treated with TNF-α (100 ng/mL) for 10 minutes. Bay 11-7082 (Bay 11; 1 nM), a pharmacological inactivator of NF-κB signaling, was used as an internal control of the immunoblotting analyses to show that TNF-α-induced NF-κB signaling was reduced when NF-κB signaling was pharmacologically inactivated in HGKs.TNF-α: tumor necrosis factor-alpha, NF-κB: nuclear factor kappa B, HGK: human gingival keratinocyte, GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
|
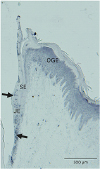 | Figure 11VDR is expressed in the gingival epithelium. VDR expression in gingival epithelium surrounding rat mandibular molars was examined using immunohistochemical methods. Expression of VDR was apparent in the JE (arrows). Expression of VDR was also localized in the OGE and SE.VDR: vitamin D receptor, JE: junctional epithelium, OGE: oral gingival epithelium, SE: sulcular epithelium.
|
DISCUSSION
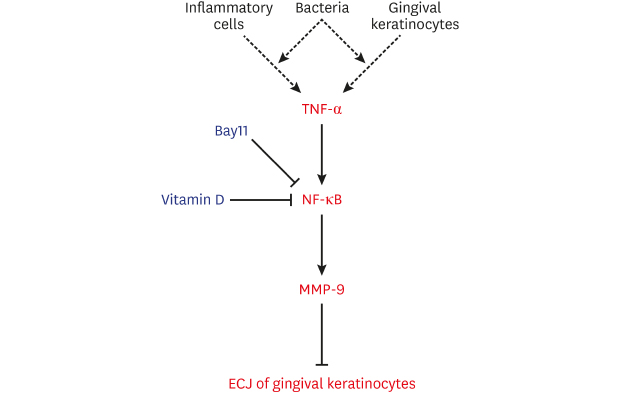
 | Figure 12Diagram of a hypothetical molecular mechanism by which vitamin D suppresses the dissociation of ECJs induced by TNF-α. Vitamin D suppresses the NF-κB activation induced by TNF-α, which abolishes the detrimental action of TNF-α through downregulating the production of MMP-9, which breaks the integrity of ECJs. TNF-α may be produced by gingival keratinocytes infected with bacteria in an autocrine fashion at the early epithelial stage of infection [7] or could be externally provided from inflammatory cells in connective tissue at the late stage of inflammation. In summary, the present study suggests that vitamin D may be protective for periodontal health by strengthening the epithelial barrier.ECJ: E-cadherin junction, TNF-α: tumor necrosis factor-alpha, NF-κB: nuclear factor kappa B, MMP-9: matrix metalloproteinase-9.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download