Abstract
Rosmarinic acid (RA) is a natural polyphenolic compound that exists in many medicinal species of Boraginaceae and Lamiaceae. The previous studies have revealed that RA had therapeutic effects on hepatocellular carcinoma (HCC) in the H22-xenograft models by inhibiting the inflammatory cytokines and NF-κB p65 pathway in the tumor microenvironment. However, its molecular mechanisms of immunoregulation and pro-apoptotic effect in HCC have not been fully explored. In the present study, RA at 75, 150, and 300 mg/kg was given to H22 tumor-bearing mice via gavage once a day for 10 days. The results showed that RA can effectively inhibit the tumor growth through regulating the ratio of CD4+/CD8+ and the secretion of interleukin (IL)-2 and interferon-γ, inhibiting the expressions of IL-6, IL-10 and signal transducer and activator of transcription 3, thereby up-regulating Bax and Caspase-3 and down-regulating Bcl-2. The underlying mechanisms involved regulation of immune response and induction of HCC cell apoptosis. These results may provide a more comprehensive perspective to clarify the anti-tumor mechanism of RA in HCC.
Hepatocellular carcinoma (HCC) is one of the most common liver malignancies and has become the second leading cause of cancer death in China [1]. Although various drugs are used for HCC treatment, these drugs can only provide very limited treatment efficacy and usually have serious adverse effects [2]. Thus, it is essential to develop new drugs that have a better efficiency and less side effects for HCC.
Nowadays, traditional herbal medicine and molecular compounds derived from the herbs are showing their high efficacy in HCC treatment. Among them, rosmarinic acid (RA), a natural polyphenolic compound, is a potential candidate. It is abundant in and thus can be extracted from many herbal medicinal species of Boraginaceae and Lamiaceae, such as rosemary and purple perilla. Recent studies have revealed that RA possessed antioxidant, anti-inflammatory, and anti-angiogenic effects [34]. Furthermore, accumulating evidence indicates that RA has anti-tumor effects by inhibiting tumor cell growth [5]. Our previous studies showed that RA had anti-tumor effects in the H22-xenograft models with little toxicity effects and the mechanism involved inhibition of inflammatory cytokines and NF-κB p65 pathway in the tumor microenvironment [6]. Its high efficacy in inhibiting tumor cell growth and minimal toxicity to immune organs, make it a potential drug for HCC treatment. Yet, the underlying mechanisms of immunoregulation and pro-apoptotic effect in HCC are poorly understood.
The liver is an immunologically unique organ with necessary capabilities for the development of immune responses. Unresolved immune responses play a crucial role in promoting the growth and progression of HCC [78]. Furthermore, the pathologic progress of HCC is tightly related to the dysregulation of cell apoptosis, including activation of signal transducer and activator of transcription 3 (STAT3) pathway and the Bcl-2 family [910]. Therefore, it is essential to clarify the underlying mechanisms of RA on HCC from the perspective of immunoregulatory and apoptosis. In the present work, to fully explore the anti-tumor mechanisms of RA, we focused specifically on elucidating the immunoregulatory activity and pro-apoptotic effect of RA in H22 tumor-bearing mice.
Specific Pathogen Free (SPF) male Kunming mice weighting ranging from 18 to 22 g were purchased from Experimental Animal Center of Guangxi Medical University (Guangxi, China). The animals were housed at conditions of 25 ± 2℃ and humidity of 60 ± 10% with 12 h light-on and 12 h light-off. The H22 HCC cell line was provided by China Center for Type Culture Collection. The animal experimental procedure was approved by the Institutional Animal Ethical Committee of Guangxi Medical University. The certificate number of animal care is SYXY GUI 2014-0003.
Seven days after H22 cell inoculation into the peritoneal cavity of the mice, ascites was extracted from the tumor-bearing mice and diluted to the concentration of 1.5 × 107/ml with PBS. Then, 0.2 ml cell suspension was inoculated subcutaneously into the right side of the axillary of each mouse. One day after the inoculation, the tumor-bearing mice were randomly divided into 5 groups with 6 mice per group. A group of mice were injected with the same amount of normal saline, but not the H22 cells, as non-tumor controls. The positive control group received cyclophosphamide (CTX; Shanxi Powerdone Pharmaceutics Co., Ltd., Shanxi, China) at a dose of 20 mg/kg once every two days via intra-peritoneal injection whereas the RA receiving group administered via gavage once a day escalating (75, 150, and 300 mg/kg) doses of rosmarinic acid (purity > 98%; Chengdu must biotechnology Co., Ltd., Chengdu, China) for 10 days. Model group and non-tumor group received normal saline at a dose of 20 ml/kg. All the animals were euthanized the next day after the last treatment. The body weights of all the animals were measured. Blood samples, xenograft tumors, thymus and spleen of the animals were collected for further analyses.
The rate of tumor growth inhibition and immune organ index were calculated by the following formula:
Tumor growth inhibition rate (%) = (WModel − WTreated) / WModel × 100, in which WModel was the tumor weight of the untreated mice whereas WTreated was the mean tumor weight of treated mice;
Immune organ index was the organ weight (mg) divided by body weight (g).
The collected anticoagulated blood was stained with antimouse CD4 FITC, CD8a PE and CD3e PE-Cyanine5 in the dark for 30 min and incubated with lysing solution for FACS for 10 min. After washing three times with lysing cytometry staining buffer, the percentages of CD3+, CD4+ and CD8+ T lymphocytes were determined by FACS C5 flow cytometry (Becton Dickinson, Cockeysville, MD, USA) and analyzed using BD Accuri C6 Software.
Frozen xenograft tumor was washed with saline and then homogenized with saline on ice. Homogenates were centrifuged at 3,000 g for 10 min at 4℃ and the supernatants were collected. Levels of interleukin (IL)-2, Interferon-γ (IFN-γ), IL-6 and IL-10 were measured by enzyme linked immunosorbent assays (ELISA) kits (Ebioscience, San Diego, CA, USA) according to manufacturer's instruction.
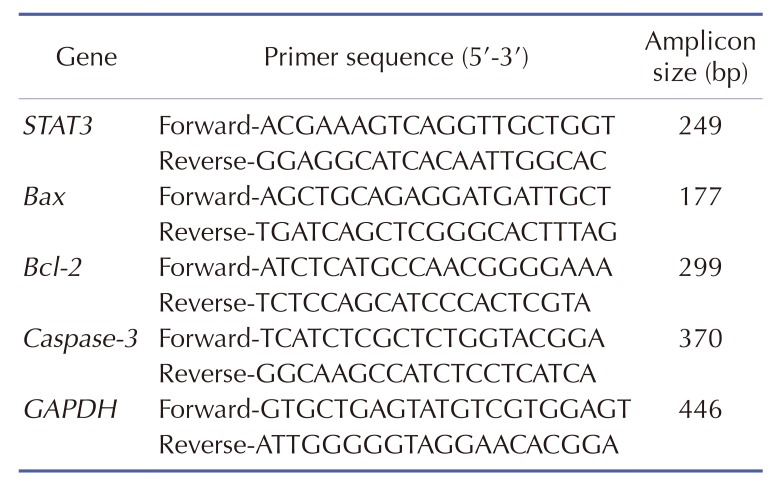
All xenograft tumors were dissected out and fixed with 10% buffered formalin and embedded in paraffin. Sections of 5 µm in thickness were prepared and stained with immunohistochemistry. Immunohistochemical detection of STAT3, Bax, Bcl-2 and Caspase-3 was performed by using the PV kit (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) according to the manufacturer's instructions. The sections were incubated with anti-STAT3 (Bioworld, Louis Park, MN, USA), anti-Bax, anti-Bcl-2 and anti-Caspase-3 (Boster Biological Technology Co., Ltd., Wuhan, China) overnight at 4℃. Counterstaining was performed using hematoxylin.
Total RNA was extracted from the tumor samples using Trizol-Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instruction. RNA was reverse transcribed to cDNA using a reverse transcriptase reaction kit (Fermentas, Hanover, MD, USA). The qRT-PCR conditions were as follows: 95℃ for 10 min, follow by 40 cycles of 95℃ for 15 sec, 60℃ for 60 sec. The gene names (including STAT3, Bax, Bcl-2, Caspase-3 and GAPDH), primer sequence, and amplifying size are listed in Table 1. GAPDH was used as a loading control. Data were analyzed by the method of 2−ΔΔCt.
All data were presented as mean ± standard deviation. Statistical analysis was performed with SPSS 20.0 software (IBM Co., Armonk, NY, USA). One-way analysis of variance (ANOVA) followed by Dunnett's t-test was used for statistical analyses, with p < 0.05 set for statistical significance.
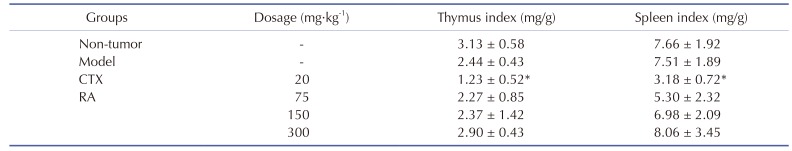
The tumor weights and animal body weights were measured after 10 days of treatment (Table 2). The average tumor weight of model group was 2.29 ± 0.79 g but that of groups treated with CTX and with 300 mg/kg RA were 1.15 ± 0.27 and 1.24 ± 0.07, respectively (p < 0.05), which could be converted to growth inhibition rates of 48.87% and 44.98%, respectively. These results suggested that the treatment with CTX and 300 mg/kg RA could effectively inhibit the tumor growth in the mice-bearing H22 tumors. Furthermore, CTX could inhibit the thymus and spleen indexes of tumor-bearing mice compared with model group (p < 0.05), whereas RA treatment had little effect on the immune indexes (Table 3), indicating lower toxicity to immune organs than CTX.
Compared with the model group, RA treatment did not obviously change the percentages of CD3+ and CD4+ T lymphocytes of peripheral blood (Fig. 1A–D). Treatment with 75 mg/kg and 150 mg/kg RA significantly decreased the percentage of CD8+ T lymphocyte whereas CTX increased it (Fig. 1E, p < 0.05). Furthermore, 300 mg/kg RA treatment caused an increment in the ratio of CD4+/CD8+ (Fig. 1F, p < 0.05). These results suggested that RA plays a vital role in immune system by regulating the ratio of lymphocytes subsets.
As shown in Fig. 2, treatment with 75 mg/kg RA elevated levels of IL-2 and IFN-γ in xenograft tumor, and the high-dose RA significantly increase IL-2 level (Fig. 2A, B; p < 0.05). Compared with the model group, treatment with CTX or 300 mg/kg RA significantly hampered the elevation of IL-6 and IL-10 (Fig. 2C, D; p < 0.05). Collectively, these results suggested that RA plays a part in immune system by regulating the secretion of immunoregulatory cytokines.
Immunohistochemistry analysis showed that the CTX group and RA (75, 150, 300 mg/kg) treatment groups decreased the expression of STAT3 protein (Fig. 3A, B; p < 0.05). qRT-PCR results showed that the expression of STAT3 mRNA was decreased after CTX, 150 mg/kg and 300 mg/kg RA treatment compared with model group (Fig. 3C, P<0.05). These data suggested that the anti-tumor effects of RA may be mediated by down-regulation of STAT3 protein.
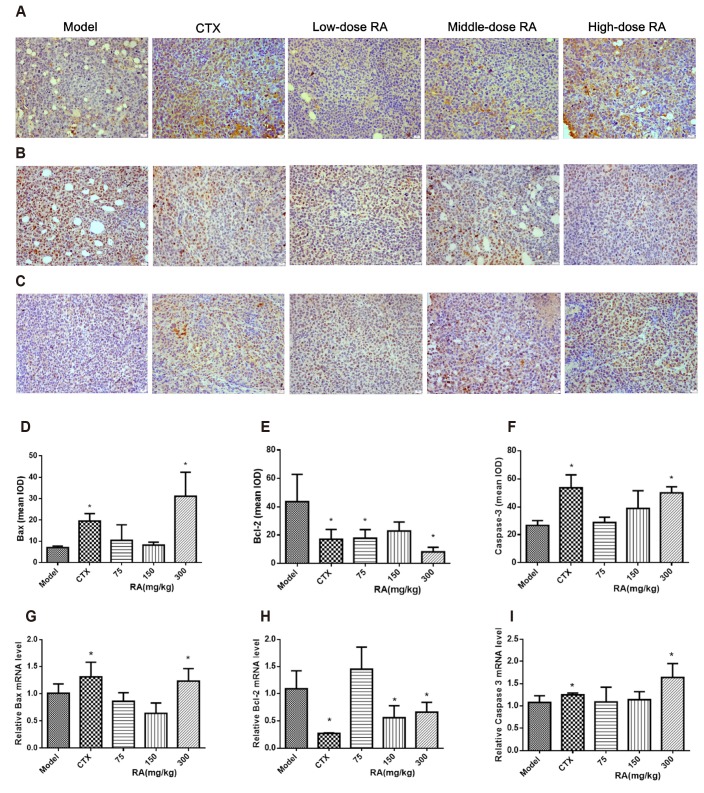
Immunohistochemistry analysis showed that treatment with CTX or 300 mg/kg RA significantly increased Bax and Caspase-3 protein expressions compared with model group. The expression of Bcl-2 protein was decreased after CTX, 75 mg/kg and 300 mg/kg RA treatment (Fig. 4A–F; p < 0.05). qRT-PCR results also showed that CTX or 300 mg/kg RA treatment increased Bax and Caspase-3 mRNA expressions, and the expression of Bcl-2 mRNA was decreased after CTX, 150 mg/kg and 300 mg/kg RA treatment (Fig. 4G–I; p < 0.05). These data suggested that the RA exerted pro-apoptotic effects by up-regulating Bax and Caspase-3 and down-regulating Bcl-2.
Recent studies have shown that RA has therapeutic effects on colon carcinogenesis [11], gastric carcinoma [12], and breast carcinoma [13]. But its ability to inhibit tumor progression in the liver has hardly been reported. Our previous studies revealed that RA had therapeutic effects on HCC in the H22-xenografts model with little toxicity effects by inhibition of tumor cell growth and enhancement of tumor cell death [6]. In the present study, 10 days treatment with RA decreased the weight of xenograft tumors in HCC xenograft models. Its antitumor effect is compatible with CTX, the most widely used chemotherapy, which dramatically decreased the thymus and spleen organ indexes, indicating a severe toxicity. These data suggest that RA can effectively inhibit the tumor growth without obvious toxicity to some immune organs, thus having a therapeutic potential for HCC.
The immune system plays a role in protecting the host against tumor. The liver is an immunologically unique microenvironment with the capacity of immune response. There have been extensive studies showing that anti-tumor immune response could be activated and then inhibit tumor progression [1415]. Within the immune system, CD4+ and CD8+ T lymphocytes along with their characteristically secreted cytokines, including IFN-γ and IL-2, function as the major anti-tumor immune response. It is generally recognized that CD4+ and CD8+ T lymphocytes comprise the primary force of immune cells and are responsible for inhibiting tumor progression. Clinical studies have suggested that HCC patients usually have a significant decrease in the ratio of CD4+/CD8+, which could lead to a dysfunction of the immune system and accelerate the progression HCC [161718]. Additionally, IFN-γ and IL-2 also play important roles in preventing and suppressing the development of tumor [1920]. In this study, the ratio of lymphocytes subsets and the levels of IL-2 and IFN-γ were measured to examine the immunomodulatory activity of RA on HCC. Our results show that RA can decrease the percentage of CD8+ T lymphocyte in the lower dose (75 and 150 mg/kg), and increase the ratio of CD4+/CD8+ when the dose is up to 300 mg/kg, suggesting that high dose RA treatment can regulate the immune system in the microenvironment as a whole, thereby relieving the HCC progression. Moreover, levels of IL-2 and IFN-γ were also increased after RA treatment. These data suggest that RA plays an anti-tumor role by regulating the ratio of lymphocytes subsets and the secretion of immunoregulatory cytokines. CTX is one of the most widely used antitumor drugs of alkylation agents clinically, which has a potent killing effect to rapidly proliferating tumor cells. Yet, the application of CTX often results in leukopenia, immunosuppression, and myelosuppression. Our results showed that CTX decreased both immune organ indexes and the ratio of CD4+/CD8+, which might relate to the immunosuppression of CTX.
STAT3 is an important transcriptional factor associated with immune responses, apoptosis and tumorigenesis in the pathologic progress of HCC. Aberrant expression of STAT3 signaling can initiate the transcription of a series of anti-apoptotic genes, constituting a significant anti-apoptotic signaling [2122]. IL-6 is a one of the major STAT3-activating cytokines which promote tumor cell proliferation and inhibit their apoptosis [23]. It has been shown that elevated IL-6 level is associated with increased HCC risk [24]. IL-10, participating in the proliferation and survival of tumor cells, can also activate STAT3 and thereby promote tumor development [25]. In the present study, levels of IL-6, IL-10, and the expression of STAT3 are found to be increased in the xenograft tumor. RA can not only prevent the increases of these cytokines but also decrease the protein and mRNA levels of STAT3.
To further clarify the pro-apoptotic effect of RA on HCC, expressions of Bax, Bcl-2 and Caspase-3, which regulate the process of apoptosis in tumor, were examined. Bcl-2 family protein members including Bax and Bcl-2 are recognized as the first regulatory step for inducing mitochondrial apoptosis [26]. Bcl-2, as a downstream protein regulated by STAT3, plays pivotal roles in inhibiting pro-apoptotic signaling and thus contributing to carcinogenesis, whereas Bax is a promoter of apoptosis with diminished levels found in HCC [2728]. Apoptosis is a genetically regulated programmed cell death including the activation of Caspase-3. The overexpression of Caspase-3 has received great attention because it is a major executor for apoptosis of tumor cells and is capable of amplifying and accelerating the apoptotic process [29]. Results of the current study showed that RA treatment increased the expressions of Bax and Caspase-3 whereas decreased the expression of Bcl-2. These data collectively suggest that RA exerts pro-apoptotic effects by inhibiting of STAT3 pathway, thereby upregulating Bax and Caspase-3 and down-regulating Bcl-2.
In conclusion, our present studies show that RA has therapeutic effects on HCC in the H22-xenografts model with little toxicity to immune organs. The possible mechanism might be related to regulation of immune response, as well as apoptosis-associated cytokines and STAT3 pathway. According to our studies, the anti-tumor effects of RA on HCC could be determined by the immunoregulatory activity and pro-apoptotic effect of tumor cells. These results may provide a more comprehensive perspective for clarifying the anti-tumor mechanism of RA in HCC.
ACKNOWLEDGEMENTS
This study was supported by Doctoral Research Startup Foundation of Guangxi university of Chinese medicine (2017BS047) and The Scientific Research Project of Guangxi Health Committee (Z20190316).
Notes
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID: 25651787.

2. Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010; 30:3–16. PMID: 20175029.

3. Nabavi SF, Tenore GC, Daglia M, Tundis R, Loizzo MR, Nabavi SM. The cellular protective effects of rosmarinic acid: from bench to bedside. Curr Neurovasc Res. 2015; 12:98–105. PMID: 25578431.

4. Rocha J, Eduardo-Figueira M, Barateiro A, Fernandes A, Brites D, Bronze R, Duarte CM, Serra AT, Pinto R, Freitas M, Fernandes E, Silva-Lima B, Mota-Filipe H, Sepodes B. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin Pharmacol Toxicol. 2015; 116:398–413. PMID: 25287116.
5. Karthikkumar V, Sivagami G, Viswanathan P, Nalini N. Rosmarinic acid inhibits DMH-induced cell proliferation in experimental rats. J Basic Clin Physiol Pharmacol. 2015; 26:185–200. PMID: 25210763.

6. Cao W, Hu C, Wu L, Xu L, Jiang W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J Pharmacol Sci. 2016; 132:131–137. PMID: 27707649.

7. Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol. 2011; 11:879–889. PMID: 21241810.

8. Wirth TC. Spontaneous and therapeutic immune responses in hepatocellular carcinoma: implications for current and future immunotherapies. Expert Rev Gastroenterol Hepatol. 2014; 8:101–110. PMID: 24410473.

9. Xiang M, Su H, Hong Z, Yang T, Shu G. Chemical composition of total flavonoids from Polygonum amplexicaule and their proapoptotic effect on hepatocellular carcinoma cells: Potential roles of suppressing STAT3 signaling. Food Chem Toxicol. 2015; 80:62–71. PMID: 25754378.

10. Subramaniam A, Shanmugam MK, Perumal E, Li F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, Hui KM, Sethi G. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta. 2013; 1835:46–60. PMID: 23103770.

11. Venkatachalam K, Gunasekaran S, Jesudoss VA, Namasivayam N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp Toxicol Pathol. 2013; 65:409–418. PMID: 22236574.

12. Han S, Yang S, Cai Z, Pan D, Li Z, Huang Z, Zhang P, Zhu H, Lei L, Wang W. Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des Devel Ther. 2015; 9:2695–2703.
13. Xu Y, Jiang Z, Ji G, Liu J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010; 76:956–962. PMID: 20157877.

14. Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007; 19:203–208. PMID: 17292599.

15. Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011; 7:651–658. PMID: 21647333.

16. Ramzan M, Sturm N, Decaens T, Bioulac-Sage P, Bancel B, Merle P, Tran Van, Slama R, Letoublon C, Zarski JP, Jouvin-Marche E, Marche PN, Leroy V. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int. 2016; 36:434–444. PMID: 26215124.

17. Wu D, Wei S, Liu B, Wu X, Feng Y, Luo C, Ju Y, Liang J. Effect of immune suppression on metastasis in a patient with hepatocellular carcinoma metastasized to the colon and stomach: a case report. Exp Ther Med. 2016; 11:1741–1747. PMID: 27168796.

18. Schmidt N, Neumann-Haefelin C, Thimme R. Cellular immune responses to hepatocellular carcinoma: lessons for immunotherapy. Dig Dis. 2012; 30:483–491. PMID: 23108304.

19. Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005; 32:231–245. PMID: 16106075.
20. Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004; 28:109–123. PMID: 15473953.

21. He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011; 21:159–168. PMID: 21187858.

22. Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: a review. Int J Cancer. 2016; 138:2570–2578. PMID: 26559373.

23. Zhao H, Guo Y, Li S, Han R, Ying J, Zhu H, Wang Y, Yin L, Han Y, Sun L, Wang Z, Lin Q, Bi X, Jiao Y, Jia H, Zhao J, Huang Z, Li Z, Zhou J, Song W, et al. A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget. 2015; 6:31927–31943. PMID: 26376676.

24. Ohishi W, Cologne JB, Fujiwara S, Suzuki G, Hayashi T, Niwa Y, Akahoshi M, Ueda K, Tsuge M, Chayama K. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014; 134:154–163. PMID: 23784949.

25. Chan SL, Mo FK, Wong CS, Chan CM, Leung LK, Hui EP, Ma BB, Chan AT, Mok TS, Yeo W. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer. 2012; 118:3984–3992. PMID: 22180222.

26. Karabulut AB, Karadag N, Gurocak S, Kiran T, Tuzcu M, Sahin K. Apricot attenuates oxidative stress and modulates of Bax, Bcl-2, caspases, NFκ-B, AP-1, CREB expression of rats bearing DMBA-induced liver damage and treated with a combination of radiotherapy. Food Chem Toxicol. 2014; 70:128–133. PMID: 24819963.

27. Shao J, Meng Q, Li Y. Theaflavins suppress tumor growth and metastasis via the blockage of the STAT3 pathway in hepatocellular carcinoma. Onco Targets Ther. 2016; 9:4265–4275. PMID: 27478384.

28. Yue W, Zheng X, Lin Y, Yang CS, Xu Q, Carpizo D, Huang H, DiPaola RS, Tan XL. Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2. Oncotarget. 2015; 6:21208–21224. PMID: 26056043.

29. Ooi KL, Tengku Muhammad TS, Lam LY, Sulaiman SF. Cytotoxic and apoptotic effects of ethyl acetate extract of Elephantopus mollis Kunth. in human liver carcinoma HepG2 cells through caspase-3 activation. Integr Cancer Ther. 2014; 13:NP1–NP9. PMID: 22336595.

Fig. 1
Effects of rosmarinic acid (RA) on CD3+, CD4+, and CD8+ in tumor-bearing mice.
(A, C) Percentage of CD3+ T lymphocyte. (B, D, E) Percentages of CD4+ T and CD8+ T lymphocyte. (F) Ratio of CD4+/CD8+. Percentages of CD3+, CD4+, and CD8+ T lymphocytes were determined by flow cytometry. Data were presented as mean ± standard deviation. #p < 0.05 compared with non-tumor group, *p < 0.05 compared with model group.
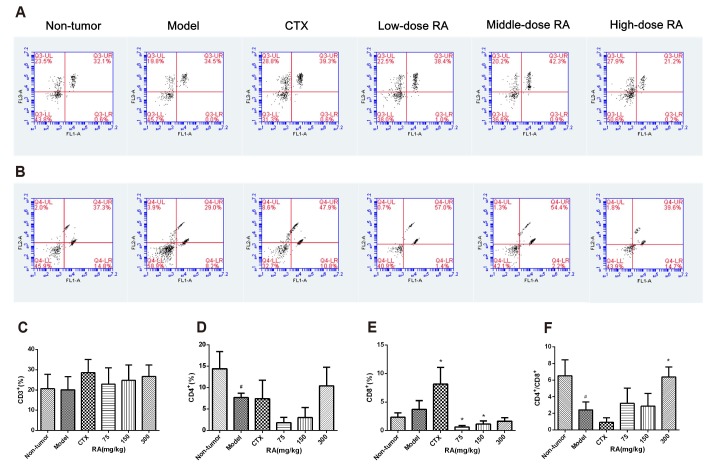
Fig. 2
Effects of rosmarinic acid (RA) on cytokine levels in xenograft tumor tissues.
tumor tissues were collected after RA treatment. Levels of interleukin (IL)-2 (A), interferon (IFN)-γ (B), IL-6 (C) and IL-10 (D) in tumor tissues were measured by ELISA. Data were presented as mean ± standard deviation. *p < 0.05 compared with model group.
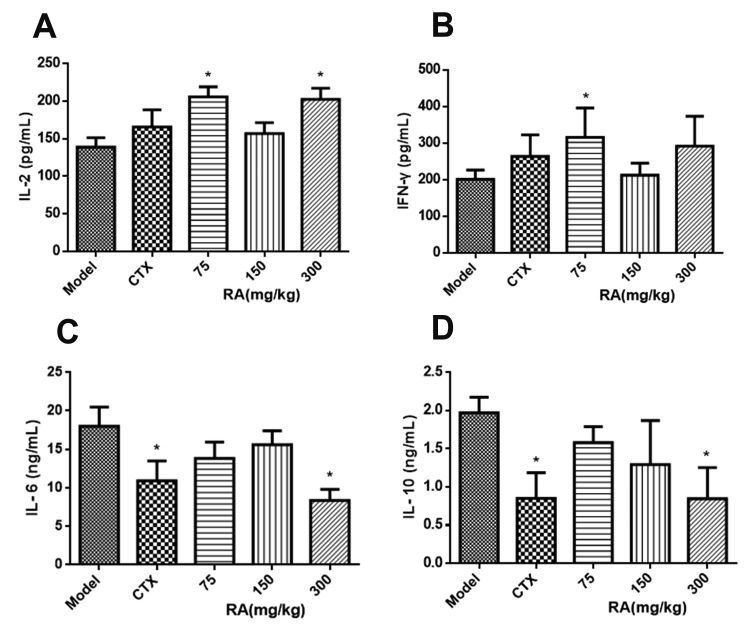
Fig. 3
Effects of rosmarinic acid (RA) on signal transducer and activator of transcription 3 (STAT3) in xenograft tumor tissues.
(A, B) At the end of RA treatment, STAT3 protein expression was measured by immunohistochemistry (magnification, ×400). The images were analyzed by an Image Pro Plus 6.0 system. (C) Total RNA was extracted from xenograft tumor tissues. The expression of STAT3 mRNA was measured by qRT-PCR. GAPDH was used as a loading control. Data were presented as mean ± standard deviation. IOD, integrated opticdensity. *p < 0.05 compared with model group.
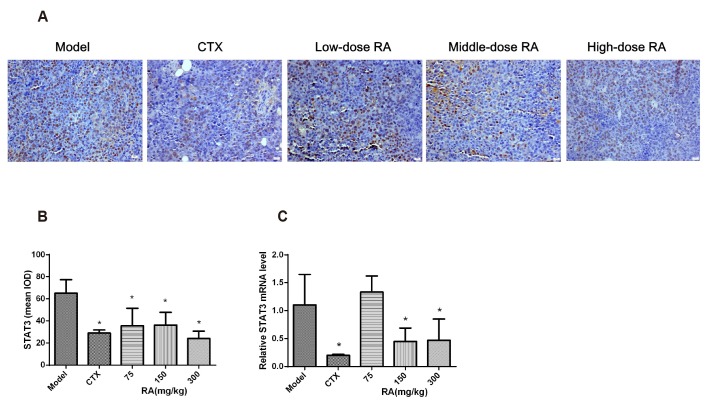
Fig. 4
Effects of rosmarinic acid (RA) on expressions of Bax, Bcl-2, and Caspase-3 in xenograft tumor tissues.
Expressions of Bax, Bcl-2, and Caspase-3 proteins were measured by immunohistochemistry (magnification, ×400). The images were analyzed by an Image Pro Plus 6.0 system. Expressions of Bax, Bcl-2, and Caspase-3 mRNA were measured by qRT-PCR. GAPDH was used as a loading control. (A, D) Bax protein expression; (B, E) Bcl-2 protein expression; (C, F) Caspase-3 protein expression. (G) Bax mRNA expression; (H) Bcl-2 mRNA expression; (I) Caspase-3 mRNA expression. Data were presented as mean ± standard deviation. IOD, integrated opticdensity. *p < 0.05 compared with model group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download