Abstract
Dipeptidyl peptidase (DPP)-4 inhibitors, or gliptins, are a class of oral hypoglycemic drugs that have been widely used as a second-line treatment for type 2 diabetes. Gliptins, which were introduced for clinical use a decade ago, have been shown to be beneficial against nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NASH) in animals and humans. Cenicriviroc (CVC), a dual antagonist of C-C chemokine receptor type 2 and 5, is currently under investigation against NASH and fibrosis. It was previously discovered that evogliptin (EVO) reduces hepatic steatosis in diet-induced obese animals but the effectiveness of EVO on NASH remains unexplored. Here, we compared the effectiveness of EVO and CVC against NASH and fibrosis in mice fed a high-fat and high-fructose diet (HFHF). Biochemical and histological analyses showed that mice fed a HFHF for 20 weeks developed severe hepatic steatosis and inflammation with mild fibrosis. Administration of EVO (0.2% wt/wt) for the last 8 weeks of HFHF feeding significantly reduced hepatic triglyceride accumulation, inflammation, and fibrosis as well as restored insulin sensitivity, as evidenced by lowered plasma insulin levels and the improvement in insulin tolerance test curves. Treatment of mice with CVC (0.1% wt/wt) inhibited hepatic inflammation and fibrogenesis with similar efficacy to that of EVO, without affecting hepatic steatosis. CVC treatment also reduced plasma insulin concentrations, despite no improvement in insulin tolerance. In conclusion, EVO administration efficiently ameliorated the development of NASH and fibrosis in HFHF-fed mice, corroborating its therapeutic potential.
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease and often progresses to nonalcoholic steatohepatitis (NASH). NASH ranges from simple steatosis to inflammation and fibrosis and can deteriorate to cirrhosis. NAFLD/NASH is usually associated with obesity-linked insulin resistance and type 2 diabetes. Due to the rapidly growing incidence of obesity and NAFLD/NASH and their huge impact on global health, the necessity for the development of novel therapeutic medicine against NASH is increasing and urgent.
Dipeptidyl peptidase (DPP)-4 inhibitors enhance the biological half-life of incretins such as glucagon-like peptide 1 by inhibiting its degrading enzyme DPP-4 and thereby increase insulin secretion from pancreatic beta cells. These inhibitors are currently used for the management of type 2 diabetes since they were first introduced around 10 years ago. DPP-4 inhibitors have also been observed to have beneficial effects for the treatment of NAFLD/NASH in animal models [1234], although their effects in humans remain controversial. For instance, sitagliptin treatment to diet-induced obese mice reduced hepatic steatosis with attenuation of gene expression associated with de novo lipogenesis, but upregulated fatty acid oxidation genes [5]. It has been shown that administration of sitagliptin 100 mg once daily for 1 year ameliorated NAFLD activity scores (NAS) by improving steatosis and ballooning, irrespective of the glucose-lowering effects against diabetes [6]. Contrary to this, another study observed that sitagliptin did not cause reduction of steatosis [78]. Evogliptin (EVO) is a selective DPP-4 inhibitor that received its first global approval in South Korea in 2015 for the treatment of type 2 diabetes [910]. A recent study has shown that EVO has a protective effect against hepatic steatosis in diet-induced obese mice [11]. However, whether EVO is effective in the prevention or treatment of NASH and fibrosis remains to be explored.
Cenicriviroc (CVC) was originally developed for the treatment of human immunodeficiency virus [1213], but has now gained attention due to its anti-fibrosis effect in mice, rats, and humans [14]. CVC acts as a dual antagonist of C-C chemokine receptors type 2 and 5 (CCR2/CCR5), which are associated with liver inflammation and fibrosis [1516]. CCR2/CCR5 and their respective ligands CCL2 or monocyte chemotactic protein (MCP)-1 and CCL5 play an important role in immune cell migration and tissue infiltration in NASH pathology. A recent report of a randomized phase 2 clinical study performed for two years indicated that CVC has the ability to reduce hepatic inflammation and fibrosis in NASH patients [17]. Therefore, to date, CVC along with obeticholic acid which is a farnesoid X nuclear receptor ligand and elafibranor which is an agonist of the peroxisome proliferator-activated receptor-α and -δ, are the only agents that have been clinically shown to improve fibrosis in NASH, while many other pharmacological treatments such as gliptins and pioglitazone are currently undergoing evaluation [1819202122].
Based on previous findings, it was hypothesized that EVO could prevent the development of NASH by influencing hepatic lipid metabolism. Experiments were subsequently performed to compare the effect of EVO with that of CVC in high-fat and high-fructose diet (HFHF)-fed mice—a well-established mild NASH model. To this end, 7-week-old C57BL/6 mice were fed a HFHF for 20 weeks and fed EVO or CVC as a diet-admixture for the last 8 weeks of HFHF feeding. It was demonstrated that EVO prevented the development of NASH in these mice by suppressing not only steatosis but also liver inflammation and fibrosis and that the latter two effects were superior to those of CVC.
EVO ((R)-4-[(R)-3-amino-4-(2, 4, 5-trifluorophenyl)butanoyl]-3-(t-butoxymethyl) piperazin-2-one) L-tartrate salt was synthesized in Dong-A ST. CVC ((S,E)-8-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-(((1-propyl-1H-imidazol-5-yl)methyl)sulfinyl) phenyl)-1,2,3,4-tetrahydrobenzo[b]azocine-5-carboxamide) methanesulfonic acid salt was gratefully provided by Allergan/Tobira Therapeutics. Primary antibodies of α-SMA (ab5694), F4/80 (ab6640) and CD11b (ab13357) were purchased from Abcam (Cambridge, UK), actin (sc-1616) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). D(-)-Fructose was purchased from Samchun Pure Chemicals (Seoul, Korea).
Six week-old male C57BL/6 mice were purchased from Samtako Bio (Osan, Korea) and acclimatized for a week. Mice were fed a normal chow diet (NCD, 13.5% fat; LabDiet, St. Louis, MO, USA) or HFHF (a 60% fat diet with 30% fructose in water) for 12 weeks and further maintained on the same diet or HFD-drug admixture for EVO (0.2% wt/wt, a target dose of 200 mg/kg/day) or CVC (0.1% wt/wt, a target dose of 100 mg/kg/day) for the further 8 weeks. At the end of 20 week-study, mice were euthanized after 6 h fasting and blood and tissues were harvested for analysis. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Woosuk University (approval No. Woosuk 2017-007).
Plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG) (Asan Pharmaceutical, Seoul, Korea), insulin (Millipore, Billerica, MA, USA) and TNFα (Invitrogen, Carlsbad, CA, USA) were measured using specific kits. For liver TG quantification, tissues were homogenized and extracted in a mixture of chloroform, methanol and distilled water (2/1/1 ratio) and liver TG contents measured using a TG assay kit were expressed as mg of TG per 100 mg of liver tissue. Insulin sensitivity was also assessed by homeostasis model assessment for insulin resistance (HOMA-IR, [26 × fasting insulin level (ng/ml) × fasting glucose level (mg/dl)/405]) [23]. DPP-4 activity in plasma and liver tissues were measured at Dong-A Socio Research Center as described previously [24].
Intraperitoneal GTT (glucose 1 g/kg of body weight) and ITT (insulin 0.75 U/kg of body weight) were performed in mice after 16 h and 6 h of fasting, respectively. The levels of blood glucose in tail vein blood sample were measured at 0, 15, 30, 60, and 120 min following glucose or insulin injection.
For histological examination, a part of liver tissue was fixed with 3.7% neutrally buffered formaldehyde and embedded in paraffin. Tissue sections (5 µm) were stained with hematoxylin and eosin (H&E) or Sirius red using standard methods for light microscopy observation. For immunohistochemical staining, sections were incubated overnight at 4℃ with antibodies against F4/80 or CD11b (Abcam) followed by incubation with secondary antibodies for 1 h at room temperature. Liver inflammation in liver biopsies was graded using a modified histologic activity index [25]. Results of stained sections were quantified by Image J (National Institute of Health, Bethesda, MD, USA) and expressed as percentage of positive cells out of total area. To assess the morphological changes in liver, NAS was determined by the degree of steatosis (0–3), lobular inflammation (0–3) and hepatocyte ballooning (0–2) according to the definition of Kleiner et al. [26].
Liver tissue lysates were prepared and western blot analysis was performed as described previously [27]. Briefly, liver tissues were homogenized in tissue protein extraction reagent (Thermo, Waltham, MA, USA) supplemented with inhibitors of proteinase and phosphatase. Protein concentrations in tissue lysates were determined by a Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA). The aliquots of lysates were separated by 6% or 12% sodium dodecyl sulfate-polyacrylamide gels electrophoresis. The separated proteins were transferred to nitrocellulose membranes. The membranes were blocked for 30 min at room temperature in Tris-buffered saline containing 1% Tween 20 and 4% skim milk and incubated with the primary antibodies against interest proteins, followed by incubation with secondary antibodies. Immunoreactive protein was visualized by ECL chemiluminescence detection kit (Amersham Biosciences, Buckinghamshire, UK). Image was obtained using ChemiDoc XRS+ System (Bio-rad, Hercules, CA, USA).
Total RNA was extracted from liver tissues with TRIzol reagent (Invitrogen). First-strand cDNA was generated using the random hexamer primer provided in the first-strand cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA). Specific primers for each gene were designed using qPrimerDepot (https://pga.mgh.harvard.edu/primerbank/) and described in Table 1. qPCR was performed as previously described using an ABI7000 and Stratagene3000 MXP PCR cycler with the SybrGreen detection system 6. The mRNA expression of all genes tested is normalized to the Rps3 gene expression.
The values presented are expressed as the mean ± standard deviation (SD). The statistical significance of the differences between treatment groups was determined by one-way ANOVA followed by Fisher's post-hoc analysis using GraphPad Prism 5.2 (San Diego, CA, USA). A p-value of less than 0.05 was considered significant.
C57BL/6 mice were fed a NCD or HFHF with or without EVO or CVC for a total of 20 weeks (drug treatment for the last 8 weeks). Body weight and food and water intake were measured weekly. Body weight was significantly higher in mice fed HFHF than in mice fed NCD during the entire drug treatment period, but decreased in mice treated with EVO despite no change in food intake (Table 2). Given obesity accompanies insulin resistance, we performed GTT and ITT 8 weeks after drug administration. Unexpectedly, glucose tolerance was preserved in the HFHF group (Fig. 1A). However, insulin resistance as assessed by ITT developed in the HFHF control group, but was ameliorated by EVO treatment (Fig. 1B). EVO also markedly decreased plasma levels of insulin and consequently HOMA-IR, a surrogate assessment of insulin resistance, compared to those of the HFHF control group (55.52 vs. 22.77) (Fig. 1C and Table 2). Although GTT and ITT were not altered by CVC treatment, plasma insulin levels were significantly decreased by CVC treatment compared to those in the HFHF control group (2.08 vs. 4.20 ng/ml) with the inhibition of HOMA-IR. These results indicate that EVO has a consistent effect in improving insulin sensitivity in various diabetes models and that CVC may have potential to reduce hyperinsulinemia in subjects with elevated basal plasma insulin levels.
After 8-week treatment with EVO or CVC, mice were sacrificed and tissue weight and blood parameters were analyzed. Liver weight and TG amounts were higher in HFHF control mice but were decreased following EVO treatment (Table 2 and Fig. 2A), suggesting that hepatic lipid accumulation was attenuated by EVO. Indeed, H&E staining of liver sections indicated that fat accumulation in the liver was elevated by the HFHF but was significantly reduced in EVO-treated mice (Fig. 2B). CVC-treated mice did not show any difference in liver fat accumulation as assessed using liver TG levels and H&E staining readout. Immunohistochemistry revealed increased accumulation of F4/80+ and CD11b+ cells in liver of mice fed HFHF whereas normalization with EVO or CVC treatment (Fig. 2B).
The plasma level of TNFα, a highly pro-inflammatory cytokine that plays a critical role in liver inflammation, was measured. HFHF increased TNFα levels by 7-fold but co-treatment with EVO reduced TNFα levels to those observed in lean NCD-fed mice (Fig. 2C). Moreover, mRNA expression of interleukin (IL)-6 (Il6) and MCP-1 (Ccl2) as well as TNFα (Tnfa) was significantly reduced in EVO-treated mice compared to that in HFHF control mice (Fig. 2D). Liver tissues from HFHF-fed mice exhibited increased macrophage infiltration, but EVO markedly decreased this (Fig. 2B–D) by reducing proinflammatory cytokine and chemokine levels. Reduced hepatic inflammation by EVO also caused a significant decrease in plasma ALT levels (Table 2), which were increased in HFHF controls, indicating hepatocyte injury in these mice. As expected, CVC treatment also reduced plasma levels of TNFα, expression of IL-6 and MCP-1, and macrophage infiltration, confirming its anti-inflammatory role in NASH. However, plasma ALT and AST levels did not differ between CVC-treated and HFHF control mice.
The hallmarks of nonalcoholic steatohepatitis are accumulation of lipid droplet, lobular inflammation and ballooning degeneration of hepatocytes. Liver TG contents and H&E-stained liver sections showed HFHF-fed mice demonstrated hepatic steatosis, immune cell infiltration, and hepatocellular ballooning in HFHF-fed control mice, thereby elevating NAS (Figs. 2A, B, and 3). However, the NAS value was significantly lower in the EVO group, confirming the role of EVO in the prevention of NASH progression. CVC also reduced NAS compared to that of the HFHF control group, but to a lesser extent than EVO.
Sirius Red staining was used to assess whether EVO treatment improved liver fibrosis. Compared with NCD mice, HFHF control mice showed collagen deposition increase by more than 6-fold (Fig. 2B), but EVO treatment reversed this effect. These findings were further supported by the increase in the levels of collagen 1 (Col2a1) mRNA and α-SMA protein, markers of activated hepatic stellate cell (HSC), in HFHF controls; these effects were significantly reduced by EVO treatment as well (Fig. 2D, E). CVC significantly reduced Sirius red-positive area and α-SMA expression to a similar degree as EVO.
In this report, using a HFHF-induced murine NASH model with fibrosis and insulin resistance, it was shown that EVO not only improves insulin sensitivity but also ameliorates NASH and fibrosis with a superior efficacy to that of CVC, an anti-NASH/fibrosis drug under clinical evaluation. It was also confirmed that EVO significantly prevented HFHF-induced hepatic steatosis, which is consistent with a previous study using HFD-fed mice [11]. In contrast to EVO, CVC did not show a pronounced improvement in steatosis as observed in the HFHF control group, although it ameliorated hepatic inflammation and fibrosis. These results are somewhat unexpected because the previous study has reported that CVC treatment (20 and 100 mg/kg/day, p.o.) in mice with NASH improved NAS with reduction in steatosis as well as lobular inflammation and hepatocyte ballooning [28]. These differences might be derived from multiple reasons such as different models of NASH (streptozotocin injection plus HFD feeding vs. HFHF), duration of HFD feeding and drug treatment (total 5 week HFD feeding with 3 week of CVC treatment vs. total 20 week HFHF feeding with 8 week of CVC treatment).
Similarly to EVO, HOMA-IR was also improved by CVC due to lowered plasma insulin levels, which has not been demonstrated earlier. Although DPP-4 inhibitor members have been shown to reduce liver steatosis, inflammation, or fibrosis in various models, this study is the first to directly compare the effect a DPP-4 inhibitor with that of a clinically proven anti-fibrotic agent. It is proposed that EVO exerts beneficial effects against NASH and liver fibrosis with better efficacy to that of CVC in HFHF-fed mice.
NASH has a very complex pathogenesis, developing from simple steatosis and characterized by hepatocyte death, inflammation, and varying degree of fibrosis [29]. Multiple mechanisms are involved in the progression from simple steatosis to NASH or NASH-associated fibrosis: (i) effects of inflammatory cytokines and chemokines, mainly those derived from obese and inflamed adipose tissue; stimulation of hepatic inflammation; (ii) production of reactive oxygen species from accumulated hepatic lipid promotes metabolic dysfunction, lipotoxicity; and (iii) obese gut leaks lipopolysaccharides (LPS) from the gut microbiome, which aggravates hepatic inflammation, lipid accumulation, hepatocyte apoptosis, and HSC activation. In the current study, it was found that EVO reduced body weight gain (approximately 8% less than that of HFHF control mice) without change in food intake, being consistent with previous results [11]. Thus, adipose-derived proinflammatory cytokines and chemokines such as TNFα, IL-6, and MCP-1 were markedly reduced in the plasma or liver by EVO administration. Consequently, TNFα stimulation of liver inflammation may be suppressed, which is reflected by reduced NAS. The impact of TNFα on NASH development and progression is very diverse, leading to apoptosis and impairment of insulin signaling in hepatocytes and also the activation of immune cells and HSC. In this study, complete normalization of plasma levels of TNFα by EVO treatment might prevent not only hepatic inflammation, insulin resistance, and hepatocyte apoptosis but also HSC activation, and thus fibrogenesis. A recent study showed that vildagliptin inhibited DPP-4 activity in gut microbiota leading to improvement in hepatic immunity by suppressing LPS production from gut microbiota [30]. As DPP-4 activity both in the plasma and the liver was completely repressed in mice treated with EVO (Table 2), it is possible that DPP-4 activity in gut microbiota and thus LPS production might be also suppressed in these mice, which could contribute to the protection from hepatic inflammation.
It was also observed that hepatic accumulation of F4/80-positive macrophages was almost completely normalized by EVO with less CD11b-positive infiltrates. This indicates reduced infiltration of circulating monocytes into liver tissues, and is attributed to less chemokine stimulation in combination with repressed activation of Kupffer cells, which may contribute to the prevention of liver inflammation. The infiltration of immune cells and subsequent inflammation are key steps to induce or modify fibrogenesis. For instance, deficiency of CCR2 or CCR5 has been shown to prevent NASH or fibrosis [1631]. In this regard, CVC as a CCR2/5 antagonist has been shown to have anti-fibrotic activity in various animal models and patients [1728]. CVC-mediated CCR2 antagonism is thought to reduce recruitment, migration, and infiltration of circulating monocytes and macrophages at the site of liver injury [3233], whereas CCR5 antagonism is expected to additionally impair the migration, activation, and proliferation of collagen-producing HSC [34].
Recently, CVC proved to be effective in reducing biomarkers of liver inflammation and fibrosis and to be safe and tolerable in a phase 2b clinical study involving subjects with NASH and fibrosis [17]. In this clinical trial, although liver fibrosis as assessed by biopsy was significantly improved, the primary endpoint i.e., ≥ 2-point improvement in NAS was not met by CVC treatment. These results indicate that benefits of CVC treatment would be greater in those with higher disease activity or fibrosis stage. Our current results reveal an unexpected function of CVC in reducing plasma levels of insulin, which were elevated by HFHF, and thus improving HOMA-IR, although GTT and ITT levels were not altered compared to those in HFHF controls. Given the heightened attention on CVC as a new anti-fibrotic agent and the common complication of hyperinsulinemia in diabetic patients with NASH, this provides an important therapeutic implication.
In conclusion, EVO treatment ameliorated hepatic steatosis, inflammation, and fibrosis in mice with NASH with superior effects to those of CVC, a promising candidate for the treatment of liver fibrosis. Considering the urgent need to develop novel therapeutic agents against NASH and fibrosis, the current findings warrant further investigation using additional doses and experimental models.
Figures and Tables
Fig. 1
Glucose tolerance test (GTT) and insulin tolerance test (ITT) in mice fed a normal chow diet (NCD) or high-fat and high-fructose diet (HFHF) with or without evogliptin (EVO) or cenicriviroc (CVC).
(A) GTT (1 g/kg glucose i.p.) and (B) ITT (0.75 U/kg insulin i.p.) were performed at 8 weeks after drug administration in mice. Area under the curve (AUC) were shown. (C) Plasma levels of insulin were analyzed by ELISA. Values are mean ± SD; n = 9–12 per group. ***p < 0.001 vs. NCD; #p < 0.05 and ##p < 0.01 vs. HFHF.

Fig. 2
Liver histology and gene expression analysis for fibrosis and inflammatory genes.
(A) Triglyceride (TG) concentrations in liver and plasma were measured with a specific TG assay kit (n = 10). (B) Representative microphotographs of liver sections stained with hematoxylin eosin (H&E), Sirius red or antibodies against F4/80 or CD11b. The number of F4/80-positive cells were counted and expressed as a percentage of total hepatocytes (n = 4). The Sirius red–positive area was determined with Image J (n = 4). Original magnification ×200. Scale bars = 250 µm. (C) Plasma levels of TNFα were measured using specific ELISA kits (n = 10). (D) mRNA expression for inflammatory genes, cytokines and chemokines was determined by qPCR (n = 9–12 per group). (E) Expression level of α-smooth muscle actin (α-SMA) protein was determined by western blot in liver tissues. Representative blot and quantification results are shown (n = 9–12 per group). NCD, normal chow diet; HFHF, high-fat and high-fructose diet; EVO, evogliptin; CVC, cenicriviroc. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. NCD; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. HFHF.
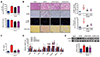
Fig. 3
Nonalcoholic fatty liver disease (NAFLD) activity scores (NAS) was determined by the degree of steatosis, lobular inflammation and hepatocyte ballooning after 8 week feeding of evogliptin (EVO) or cenicriviroc (CVC) in 20 week high-fat and high-fructose diet (HFHF) study.
Data are mean ± SD (n = 9 per group). **p < 0.01 vs. NCD; ##p < 0.01 vs. HFHF.

Table 2
Body weight, food intake, liver weight, and plasma biomarkers

Seven week old C57BL/6 mice were fed NCD or HFHF for 20 weeks. Mice treated with drug were fed on high-fat diet admixture containing EVO (0.2% wt/wt) or CVC (0.1% wt/wt) for the last 8 weeks of HFHF feeding. Results are expressed as mean ± SD (n = 9–12/group). NCD, normal chow diet; HFHF, high-fat and high-fructose diet; EVO, evogliptin; CVC, cenicriviroc; HOMA-IR, homeostasis model assessment for insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase. **p < 0.01 and ***p < 0.001 vs. NCD; ##p < 0.01 and ###p < 0.001 vs. HFHF control.
ACKNOWLEDGEMENTS
This work was supported by grants from Dong-A ST Co., Ltd. and in part by grants from the Basic Science Research Program (2017R1A2B4008593) through the National Research Foundation (NRF), which is funded by the Korean government (MSIP). We thank Dr. Mi-Kyung Kim at Dong-A ST Co., Ltd. for technical support and also thank professor Byung-Hyun Park at Chonbuk National University Medical School for discussion.
Notes
References
1. Jung YA, Choi YK, Jung GS, Seo HY, Kim HS, Jang BK, Kim JG, Lee IK, Kim MK, Park KG. Sitagliptin attenuates methionine/cholinedeficient diet-induced steatohepatitis. Diabetes Res Clin Pract. 2014; 105:47–57.

2. Klein T, Fujii M, Sandel J, Shibazaki Y, Wakamatsu K, Mark M, Yoneyama H. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol. 2014; 47:137–149.

3. Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012; 75:240–244.
4. Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch Pharm Res. 2016; 39:1114–1128.

5. Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, Amo K, Aoki K, Morimoto C, Takeda E, Terauchi Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011; 60:1246–1257.

6. Alam S, Ghosh J, Mustafa G, Kamal M, Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepat Med. 2018; 10:23–31.

7. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, Haufe W, Hooker C, Brenner DA, Sirlin CB, Loomba R. Sitagliptin vs. placebo for nonalcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016; 65:369–376.

8. Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den, Hoekstra T, Diamant M, van Raalte DH, Cahen DL. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016; 59:2588–2593.

9. Tan X, Hu J. Evogliptin: a new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2016; 17:1285–1293.

11. Kim MK, Chae YN, Ahn GJ, Shin CY, Choi SH, Yang EK, Sohn YS, Son MH. Prevention and treatment effect of evogliptin on hepatic steatosis in high-fat-fed animal models. Arch Pharm Res. 2017; 40:268–281.

12. Thompson M, Saag M, DeJesus E, Gathe J, Lalezari J, Landay AL, Cade J, Enejosa J, Lefebvre E, Feinberg J. A 48-week randomized phase 2b study evaluating cenicriviroc versus efavirenz in treatment-naive HIV-infected adults with C-C chemokine receptor type 5-tropic virus. AIDS. 2016; 30:869–878.

13. Klibanov OM, Williams SH, Iler CA. Cenicriviroc, an orally active CCR5 antagonist for the potential treatment of HIV infection. Curr Opin Investig Drugs. 2010; 11:940–950.
14. Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016; 47:356–365.

15. Saiman Y, Friedman SL. The role of chemokines in acute liver injury. Front Physiol. 2012; 3:213.

16. Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009; 50:185–197.

17. Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, et al. A randomized, placebocontrolled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018; 67:1754–1767.

18. Iogna Prat L, Tsochatzis EA. The effect of antidiabetic medications on non-alcoholic fatty liver disease (NAFLD). Hormones (Athens). 2018; 17:219–229.

19. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010; 362:1675–1685.

21. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015; 385:956–965.

22. Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A. GOLDEN-505 Investigator Study Group. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016; 150:1147–1159.e5.
23. Tomita K, Freeman BL, Bronk SF, LeBrasseur NK, White TA, Hirsova P, Ibrahim SH. CXCL10-mediates macrophage, but not other innate immune cells-associated inflammation in murine nonalcoholic steatohepatitis. Sci Rep. 2016; 6:28786.

24. Kim TH, Kim MK, Cheong YH, Chae YN, Lee Y, Ka SO, Jung IH, Shin CY, Bae EJ, Son MH. Hepatic role in an early glucose-lowering effect by a novel dipeptidyl peptidase 4 inhibitor, evogliptin, in a rodent model of type 2 diabetes. Eur J Pharmacol. 2016; 771:65–76.

25. Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006; 49:450–465.

26. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–1321.

27. Wang Z, Lee Y, Eun JS, Bae EJ. Inhibition of adipocyte inflammation and macrophage chemotaxis by butein. Eur J Pharmacol. 2014; 738:40–48.

28. Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, Richards T, Yoneyama H, Jenkins H, Wolfgang G, Friedman SL. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016; 11:e0158156.

29. Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015; 61:1066–1079.

30. Olivares M, Neyrinck AM, Pötgens SA, Beaumont M, Salazar N, Cani PD, Bindels LB, Delzenne NM. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018; 61:1838–1848.

31. Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009; 119:1858–1870.

32. Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, Lefebvre E, Luedde T, Hellerbrand C, Weiskirchen R, Longerich T, Costa IG, Anstee QM, Trautwein C, Tacke F. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018; 67:1270–1283.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download