1. Toshikuni N, Arisawa T, Tsutsumi M. Hepatitis C-related liver cirrhosis - strategies for the prevention of hepatic decompensation, hepatocarcinogenesis, and mortality. World J Gastroenterol. 2014; 20(11):2876–2887.

2. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006; 3(2):47–52.

3. Bang CS, Song IH. Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: systematic review and meta-analysis. BMC Gastroenterol. 2017; 17(1):46.

4. Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010; 139(5):1593–1601.

5. George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009; 49(3):729–738.

6. Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014; 384(9954):1597–1605.

7. Shin SK, Kim JH, Park H, Kwon OS, Lee HJ, Yeon JE, et al. Improvement of liver function and non-invasive fibrosis markers in hepatitis B virus-associated cirrhosis: 2-years of entecavir treatment. J Gastroenterol Hepatol. 2015; 30(12):1775–1781.

8. Zeuzem S, Mizokami M, Pianko S, Mangia A, Han KH, Martin R, et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: Prevalence and effect on treatment outcome. J Hepatol. 2017; 66(5):910–918.

9. Kao JH, Lee YJ, Heo J, Ahn SH, Lim YS, Peng CY, et al. All-oral daclatasvir plus asunaprevir for chronic hepatitis C virus (HCV) genotype 1b infection: a sub-analysis in Asian patients from the HALLMARK DUAL study. Liver Int. 2016; 36(10):1433–1441.

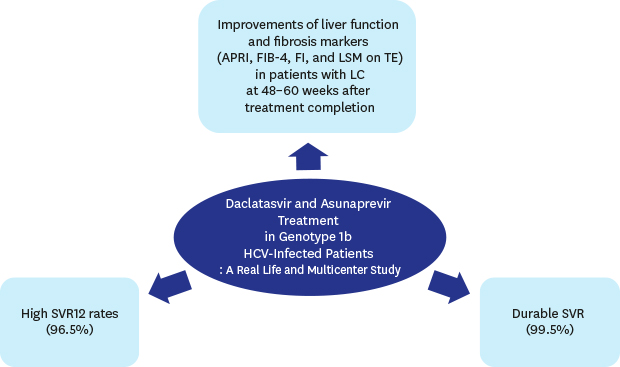
10. Yu JH, Lee JI, Lee KS, Kim JK. Real-life prevalence of resistance-associated variants against non-structural protein 5A inhibitors and efficiency of Daclatasvir + Asunaprevir therapy in Korean patients with genotype 1b hepatitis C. Virol J. 2017; 14(1):164.

11. Sezaki H, Suzuki F, Hosaka T, Akuta N, Fujiyama S, Kawamura Y, et al. The efficacy and safety of dual oral therapy with daclatasvir and asunaprevir for genotype 1b in Japanese real-life settings. Liver Int. 2017; 37(9):1325–1333.

12. Morio R, Imamura M, Kawakami Y, Morio K, Kobayashi T, Yokoyama S, et al. Safety and efficacy of dual therapy with daclatasvir and asunaprevir for older patients with chronic hepatitis C. J Gastroenterol. 2017; 52(4):504–511.

13. Ishigami M, Hayashi K, Honda T, Kuzuya T, Ishizu Y, Ishikawa T, et al. Daclatasvir and asunaprevir treatment in patients with severe liver fibrosis by hepatitis C virus genotype 1b infection: Real-world data. J Gastroenterol Hepatol. 2017; 32(11):1879–1886.

14. Itakura J, Kurosaki M, Hasebe C, Osaki Y, Joko K, Yagisawa H, et al. Complex pattern of resistance-associated substitutions of hepatitis C virus after daclatasvir/asunaprevir treatment failure. PLoS One. 2016; 11(10):e0165339.

15. McPhee F, Hernandez D, Zhou N, Ueland J, Yu F, Vellucci V, et al. Pooled analysis of HCV genotype 1 resistance-associated substitutions in NS5A, NS3 and NS5B pre-and post-treatment with 12 weeks of daclatasvir, asunaprevir and beclabuvir. Antivir Ther. 2018; 23(1):53–66.
16. Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016; 62(6):683–694.

17. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005; 115(2):209–218.

18. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol. 2016; 22(1):76–139.
19. Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014; 20(32):11033–11053.

20. van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012; 308(24):2584–2593.

21. Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018; 69(2):461–511.

22. Muir AJ, Sylvestre PB, Rockey DC. Interferon gamma-1b for the treatment of fibrosis in chronic hepatitis C infection. J Viral Hepat. 2006; 13(5):322–328.

23. Cammà C, Di Marco V, Lo Iacono O, Almasio P, Giunta M, Fuschi P, et al. Long-term course of interferon-treated chronic hepatitis C. J Hepatol. 1998; 28(4):531–537.

24. Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011; 140(7):1970–1979.

25. Ohta T, Sakaguchi K, Fujiwara A, Fujioka S, Iwasaki Y, Makino Y, et al. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama. 2006; 60(2):77–84.
26. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011; 53(3):726–736.

27. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007; 46(1):32–36.

28. Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005; 128(2):343–350.

29. Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016; 64(4):773–780.

30. Yen YH, Kuo FY, Kee KM, Chang KC, Tsai MC, Hu TH, et al. APRI and FIB-4 in the evaluation of liver fibrosis in chronic hepatitis C patients stratified by AST level. PLoS One. 2018; 13(6):e0199760.

31. European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015; 63(1):237–264.
32. Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007; 14(5):360–369.

33. Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, et al. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008; 6(9):1027–1035.









 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download