1. Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. 2009; 50:17–35.

2. Mavis AM, Alonso EM. Liver disease in the adolescent. Clin Liver Dis. 2015; 19:171–185.

3. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010; 7:425–436.

4. Bortolotti F, Guido M. Reversal of liver cirrhosis: a desirable clinical outcome and its pathogenic background. J Pediatr Gastroenterol Nutr. 2007; 44:401–406.

5. Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut. 2010; 59:861–866.

6. Pariente D, Franchi-Abella S. Paediatric chronic liver diseases: how to investigate and follow up? Role of imaging in the diagnosis of fibrosis. Pediatr Radiol. 2010; 40:906–919.

7. Kennedy P, Wagner M, Castera L, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology. 2018; 286:738–763.

8. Trout AT, Sheridan RM, Serai SD, et al. Diagnostic performance of MR elastography for liver fibrosis in children and young adults with a spectrum of liver diseases. Radiology. 2018; 287:824–832.

9. Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and metaanalysis of individual participant data. Clin Gastroenterol Hepatol. 2015; 13:440–451. e446.

10. Serai SD, Dillman JR, Trout AT. Spin-echo echoplanar imaging MR elastography versus gradient-echo MR elastography for assessment of liver stiffness in children and young adults suspected of having liver disease. Radiology. 2017; 282:761–770.

11. Kim YS, Jang YN, Song JS. Comparison of gradient-recalled echo and spin-echo echoplanar imaging MR elastography in staging liver fibrosis: a metaanalysis. Eur Radiol. 2018; 28:1709–1718.

12. Felker ER, Choi KS, Sung K, et al. Liver MR elastography at 3 T: agreement across pulse sequences and effect of liver R2* on image quality. AJR Am J Roentgenol. 2018; 211:588–594.

13. Wagner M, Corcuera-Solano I, Lo G, et al. Technical failure of MR elastography examinations of the liver: experience from a large singlecenter study. Radiology. 2017; 284:401–412.
14. Joshi M, Dillman JR, Towbin AJ, Serai SD, Trout AT. MR elastography: high rate of technical success in pediatric and young adult patients. Pediatr Radiol. 2017; 47:838–843.

15. Alustiza Echeverria JM, Castiella A, Emparanza JI. Quantification of iron concentration in the liver by MRI. Insights Imaging. 2012; 3:173–180.

16. Shin HJ, Kim HG, Kim MJ, et al. Normal range of hepatic fat fraction on dual- and triple-echo fat quantification MR in children. PLoS One. 2015; 10:e0117480.

17. Wahidiyat PA, Iskandar SD, Sekarsari D. Evaluation of iron overload between age groups using magnetic resonance imaging and its correlation with iron profile in transfusion-dependent thalassemia. Acta Med Indones. 2018; 50:230–236.
18. Serai SD, Towbin AJ, Podberesky DJ. Pediatric liver MR elastography. Dig Dis Sci. 2012; 57:2713–2719.

19. Binkovitz LA, El-Youssef M, Glaser KJ, Yin M, Binkovitz AK, Ehman RL. Pediatric MR elastography of hepatic fibrosis: principles, technique and early clinical experience. Pediatr Radiol. 2012; 42:402–409.

20. Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A. Liver fibrosis: review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017; 45:1276–1295.

21. Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010; 23:497–511.

22. Srinivasa Babu A, Wells ML, Teytelboym OM, et al. Elastography in chronic liver disease: modalities, techniques, limitations, and future directions. Radiographics. 2016; 36:1987–2006.

23. Wagner M, Besa C, Bou Ayache J, et al. Magnetic resonance elastography of the liver: qualitative and quantitative comparison of gradient echo and spin echo echoplanar imaging sequences. Invest Radiol. 2016; 51:575–581.
24. Rump J, Klatt D, Braun J, Warmuth C, Sack I. Fractional encoding of harmonic motions in MR elastography. Magn Reson Med. 2007; 57:388–395.

25. Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013; 37:544–555.

26. Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL. Hepatic MR elastography: clinical performance in a series of 1377 consecutive examinations. Radiology. 2016; 278:114–124.

27. Serai SD, Trout AT. Can MR elastography be used to measure liver stiffness in patients with iron overload? Abdom Radiol (NY). 2019; 44:104–109.

28. Kim YS, Song JS, Kannengiesser S, Seo SY. Comparison of spin-echo echoplanar imaging and gradient recalled echo-based MR elastography at 3 Tesla with and without gadoxetic acid administration. Eur Radiol. 2017; 27:4120–4128.

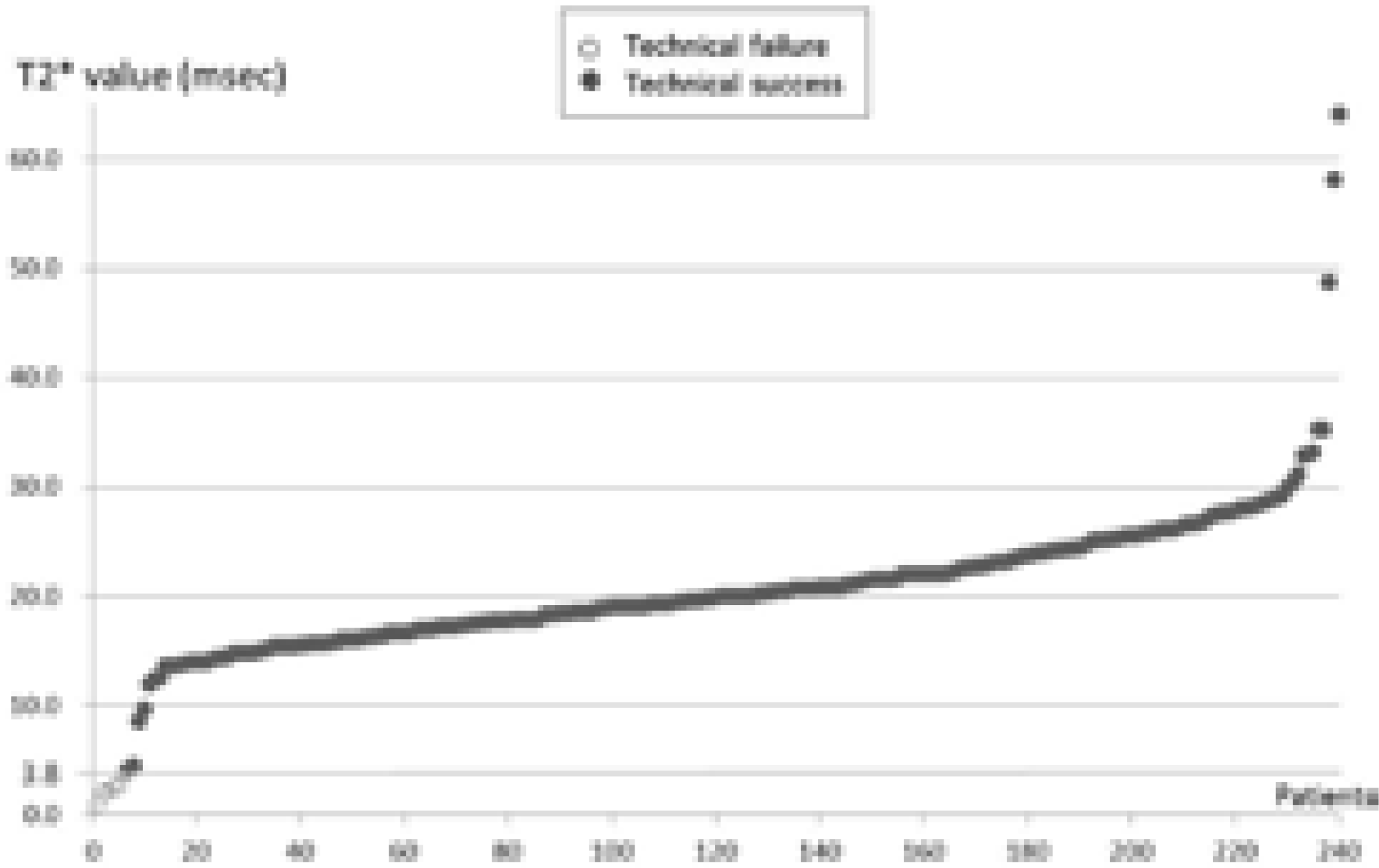




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download