INTRODUCTION
High on-treatment platelet reactivity (HPR) during clopidogrel therapy has been associated with increased rate of stent thrombosis, myocardial infarction, and mortality in several observational studies.
1)2)3)4)5) However, studies on tailored antiplatelet therapy based on the platelet function test to overcome HPR showed negative results.
6)7)8) Most of these studies used a single-time measurement of platelet function, often obtained early after administration of antiplatelet treatment with clopidogrel.
Temporal variability of platelet reactivity has recently been suggested. Analysis of Escalating Clopidogrel by Involving a Genetic Strategy–Thrombolysis In Myocardial Infarction 56 (ELEVATE-TIMI 56) trial showed significant temporal variability of platelet reactivity even though it was over a short period (<14 days).
9) However, data on long-term variability of platelet reactivity were limited. Moreover, serial changes of platelet reactivity in patients treated with ticagrelor have also rarely been evaluated. The aim of this study was to test the variability of platelet reactivity over time in patients treated with clopidogrel or ticagrelor.
METHODS
Study population
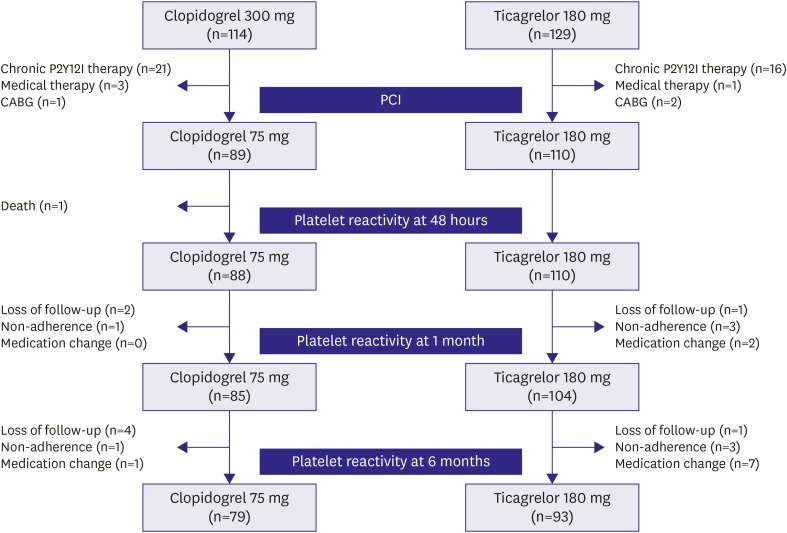
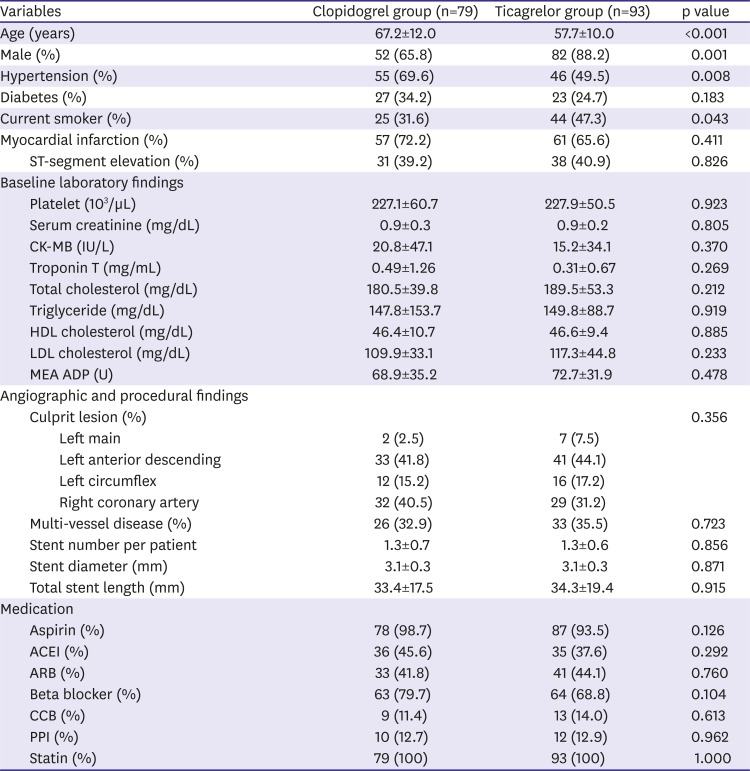
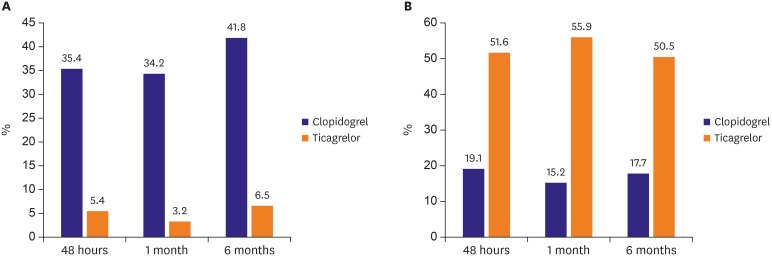
A single-center cohort of the acute coronary syndrome patients who underwent percutaneous coronary intervention (PCI) between May 2016 and December 2017 was analyzed. Inclusion criteria were patients with acute coronary syndrome treated with clopidogrel or ticagrelor, and patients who received drug-eluting stents. Exclusion criteria were patients with cardiogenic shock, chronic P2Y12 inhibitor therapy, other antiplatelet regimen except aspirin, clopidogrel, or ticagrelor, use of glycoprotein IIb/IIIa inhibitors, active hepatic disease or cirrhosis, renal failure requiring dialysis, or life expectancy <1 year. During the study period, 243 consecutive patients were administered clopidogrel or ticagrelor, followed by PCI. Among these patients, 89 patients received 300 mg loading and then 75 mg/day clopidogrel therapy and 110 patients received 180 mg loading and then 90 mg twice a day ticagrelor therapy. After excluding patients with loss of follow-up, non-adherence, or medication change due to complications or adverse events, 79 patients in the clopidogrel group and 93 patients in the ticagrelor group could complete the 6-months of follow-up (
Figure 1). Adherence to the antiplatelet agents was defined as use of more than 80% of the medication during each interval between visits, as assessed by patient self-reporting. All patients provided informed consent for processing their anonymous data according to a protocol approved by the Institutional Review Board of Wonkwang University Hospital (201603-HRE-031).
Figure 1
Study flow chart.
P2Y12I = P2Y12 inhibitor; CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention.
Percutaneous coronary intervention
Clopidogrel or ticagrelor loading was performed in the emergency room at the on-call physician's discretion. All patients received 300 mg loading of aspirin. An intravenous bolus of 5,000 U of unfractionated heparin was given, and then additional heparin boluses were administered to maintain an activated clotting time of >300 seconds during the procedure. Coronary angiography and stent implantation were performed using standard interventional techniques. Aspirin (100 mg/day), P2Y12 inhibitors and statins were prescribed to all patients after the procedure.
Measurement of platelet reactivity
For the platelet function test, blood samples were obtained from patients at baseline, 48 hours after PCI, 1 month±2 weeks, and 6 months±4 weeks. Blood sampling was performed within 8 hours after the last-dose administration.
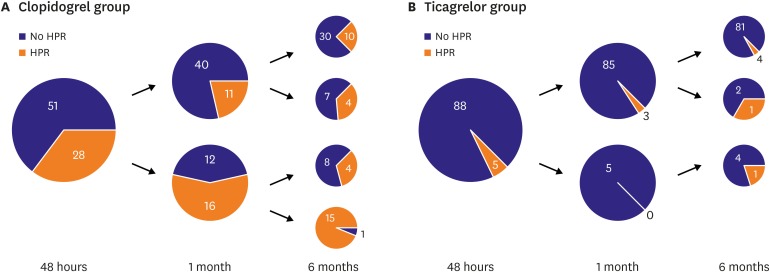
10) Platelet reactivity was measured using multiple electrode platelet aggregometry (Multiplate analyzer, Roche Diagnostic GmbH, Mannheim, Germany). The mean coefficient of variation was 5.0%, and detection range was 0~210U in our laboratory. HPR was defined as ≥47 U, and low on-treatment platelet reactivity (LPR) was defined as <19 U.
11)
Statistical analysis
Based upon a previous study, 38.2% of patients changed their clopidogrel responder status.
12) It was expected that ticagrelor treatment could reduce temporal variability. The sample size was selected to demonstrate a 20% reduction in the responder status compared with the clopidogrel group. A minimal sample size of 79 patients in each group would provide 80% power with 2-sided alpha of 0.05.
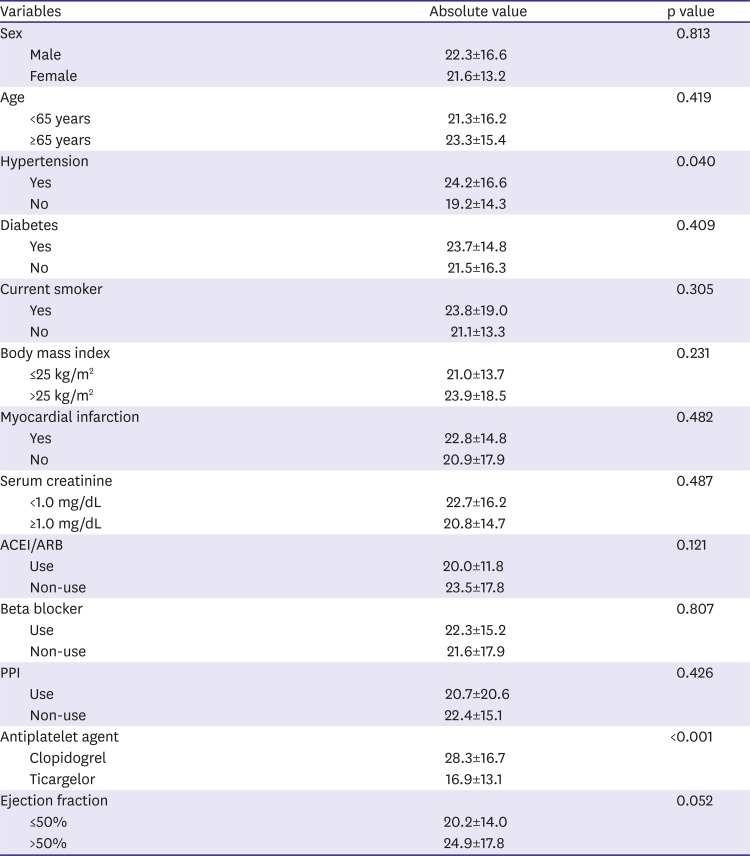
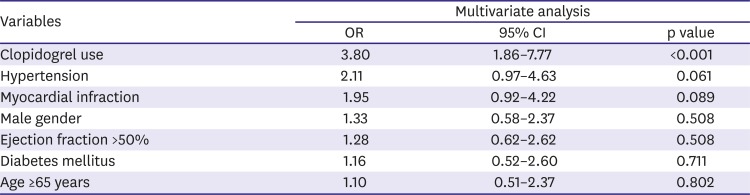
All measurements were represented as mean±standard deviation or absolute number (percentage). Inter-group analysis was performed using the independent t-test and χ2 test, which were conducted using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA). In order to compare the serial change in platelet reactivity, a paired t test was used. A multivariable logistic regression model was constructed to predict the temporal variability of platelet reactivity (greater than median value, >20 U). All p-values are two-sided, and a p-value lower than 0.05 was regarded as significant in all analyses.
DISCUSSION
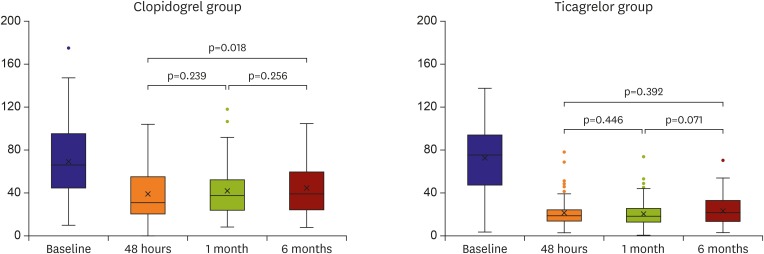
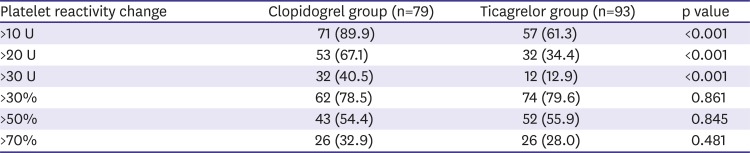
In this study, we showed high temporal variability of platelet reactivity, especially after clopidogrel treatment. Unique data about three aspects were acquired: 1) on-treatment platelet reactivity showed high intra-individual variation over time, 2) platelet reactivity did not decrease or stabilize after PCI in patients treated with clopidogrel, 3) platelet inhibition of ticagrelor treatment was stronger and relatively more stable than that of clopidogrel treatment.
After the association between HPR and increased risk of cardiovascular events was clarified, studies about tailed P2Y12 therapy, based on platelet function test, were performed. However, many studies failed to demonstrate the benefit of platelet reactivity-guided therapy. A representative study, Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis and Safety (GRAVITAS) trial randomized HPR patients to receive standard dose or high dose clopidogrel treatment.
6) Although high dose treatment resulted in a 22% reduction in HPR rate, the primary endpoint was similar between the two treatment regimens. The most likely explanation for the failure of most studies might be the low event rate of the study population and only modest increased platelet inhibition. However, as shown in this study, platelet function test at a single-time point might not reflect the platelet reactivity status of the patients.
Few studies demonstrated temporal variability of platelet reactivity. Campo et al.
13) reported that platelet reactivity decreased over time, therefore at 1 month after PCI, most baseline HPR patients showed no HPR status. The authors emphasized that one-month platelet reactivity was the strongest predictor of adverse outcomes, rather than baseline (at the time of PCI) platelet reactivity. However, sub-analysis of the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents (ADAPT-DES) showed the opposite results.
12) This study demonstrated that platelet reactivity increased over the 6 months, so the proportion of patients with HPR also increased after clopidogrel treatment. Our study showed similar results with ADAPT-DES study. Moreover, although platelet reactivity increased over time with clopidogrel treatment, ticagrelor treatment might attenuate this phenomenon.
The ADAPT-DES study showed that 38.2% of patients changed their HPR status during the 5 months standard dose clopidogrel treatment.
12) The ELEVATE-TIMI 56 trial revealed that 15.7% of patients who received standard dose clopidogrel treatment and 11.4% of patients who received double-dose clopidogrel treatment changed their HPR status within 14 days.
9) Our study showed that 43.0% of patients changed their platelet reactivity status after standard dose clopidogrel treatment. The main reason for temporal variability of platelet reactivity was not clarified. Clopidogrel is an inactive prodrug that requires in vivo conversion to an active metabolite by liver enzymes.
14)15)16) Therefore, many drug or food interactions would be expected. Moreover, sequential change of platelet reactivity after loading can be related with drug characteristics and its absorption. So, administered dose or the number of the tables of drug can influence this profile. Analysis of the ELEVATE-TIMI 56 trial suggested the presence of diabetes mellitus and body-mass index were associated with temporal variation of platelet reactivity.
9) Other potential factors might include true alterations in platelet reactivity due to fluctuations in platelet production, unrecognized noncompliance, or technical issues with the assay.
Our study has several limitations. The study was not randomized, and the sample size was small. Although serial measurements of platelet reactivity were taken prospectively, P2Y12 inhibitor therapy was not randomized. However, the aim was to demonstrate temporal variability of platelet reactivity and factors for serial platelet reactivity changes through statistical methods. We could not accurately determine the effects of temporal variability of platelet reactivity on clinical outcomes. It was not possible to fully identify concomitant medications of patients, or to confirm food and drug interactions in these patients during the study periods. Although we excluded the cases with noncompliance by patients' self-report, we did not performed tablet counting or questionnaires. We could not check time interval between last-dose administrations and measurement of platelet reactivity. One study reported weak correlation between time interval and platelet reactivity during ticagrelor treatment (r=0.25).
17) However, ONSET/OFFSET study showed that platelet reactivity on P2Y12 inhibitor, especially ticagrelor, can be variable over 8 hours after the last-dose administration.
10) In this study, all patients were suggested that blood sampling was taken in the morning after the administration of their morning pills. Therefore, platelet reactivity was measured within 8 hours after the last-dose administration in most cases. Finally, the effect of genetic polymorphism on temporal variability of platelet reactivity was unknown.
In conclusion, although ticagrelor treatment showed less temporal variability of platelet reactivity than clopidogrel treatment in terms of HPR, platelet reactivity varied over time in a significant proportion of patients. Therefore, treatment adjustment based on the platelet function test at a single-time point might be insufficient for guiding antiplatelet therapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download