1. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. HAMLET investigators. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009; 8:326–333. PMID:
19269254.

2. Fisher CM, Ojemann RG. Bilateral decompressive craniectomy for worsening coma in acute subarachnoid hemorrhage. Observations in support of the procedure. Surg Neurol. 1994; 41:65–74. PMID:
8310390.

3. Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999; 90:187–196. PMID:
9950487.

4. Honeybul S, Ho KM. How “successful” is calvarial reconstruction using frozen autologous bone? Plast Reconstr Surg. 2012; 130:1110–1117. PMID:
23096611.

5. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res. 1997; 19:311–316. PMID:
9192385.

6. Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000; 93:53–61.

7. Honeybul S. Decompressive craniectomy: a new complication. J Clin Neurosci. 2009; 16:727–729. PMID:
19261473.

8. Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003; 52:591–596. PMID:
12590683.

9. Kim JS, Cheong JH, Ryu JI, Kim JM, Kim CH. Bone flap resorption following cranioplasty after decompressive craniectomy: preliminary report. Korean J Neurotrauma. 2015; 11:1–5. PMID:
27169057.

10. Park SP, Kim JH, Kang HI, Kim DR, Moon BG, Kim JS. Bone flap resorption following cranioplasty with autologous bone: quantitative measurement of bone flap resorption and predictive factors. J Korean Neurosurg Soc. 2017; 60:749–754. PMID:
29142636.
11. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004; 100(2 Suppl Pediatrics):163–168. PMID:
14758944.

12. Lee JH, Han IH, Choi BK, Nam KH, Kim DH, Lee CS. Virtual preoperative simulation for excision of spinal tumors: surgeon processing of medical computer-assisted design software. Korean J Spine. 2017; 14:170–174. PMID:
29301180.

13. Vanaclocha V, Sáiz-Sapena N, García-Casasola C, De Alava E. Cranioplasty with autogenous autoclaved calvarial bone flap in the cases of tumoural invasion. Acta Neurochir (Wien). 1997; 139:970–976. PMID:
9401658.

14. Lee SH, Yoo CJ, Lee U, Park CW, Lee SG, Kim WK. Resorption of autogenous bone graft in cranioplasty: resorption and reintegration failure. Korean J Neurotrauma. 2014; 10:10–14. PMID:
27169026.

15. Osawa M, Hara H, Ichinose Y, Koyama T, Kobayashi S, Sugita Y. Cranioplasty with a frozen and autoclaved bone flap. Acta Neurochir (Wien). 1990; 102:38–41. PMID:
2305650.

16. Prolo DJ, Oklund SA. Composite autogeneic human cranioplasty: frozen skull supplemented with fresh iliac corticocancellous bone. Neurosurgery. 1984; 15:846–851. PMID:
6514157.

17. Dünisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C. Risk factors of aseptic bone resorption: a study after autologous bone flap reinsertion due to decompressive craniotomy. J Neurosurg. 2013; 118:1141–1147. PMID:
23451904.

18. Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 2015; 23:75. PMID:
26437934.

19. Schwarz F, Dünisch P, Walter J, Sakr Y, Kalff R, Ewald C. Cranioplasty after decompressive craniectomy: is there a rationale for an initial artificial bone-substitute implant? A single-center experience after 631 procedures. J Neurosurg. 2016; 124:710–715. PMID:
26406796.

20. Sharma M, Madhugiri V, Nanda A. James L. Poppen and surgery of the “seat of the soul”: a contemporary perspective. World Neurosurg. 2014; 82:529–534. PMID:
23403342.

21. Nair AP, Mehrotra A, Das KK, Kumar B, Srivastav AK, Sahu RN, et al. Calvarial tuberculosis of the parietal bone: a rare complication after dental extraction. Asian J Neurosurg. 2015; 10:219–221. PMID:
26396611.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



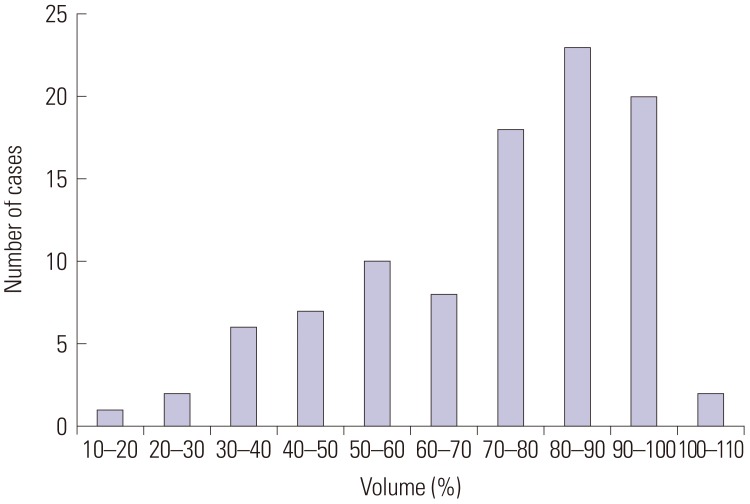
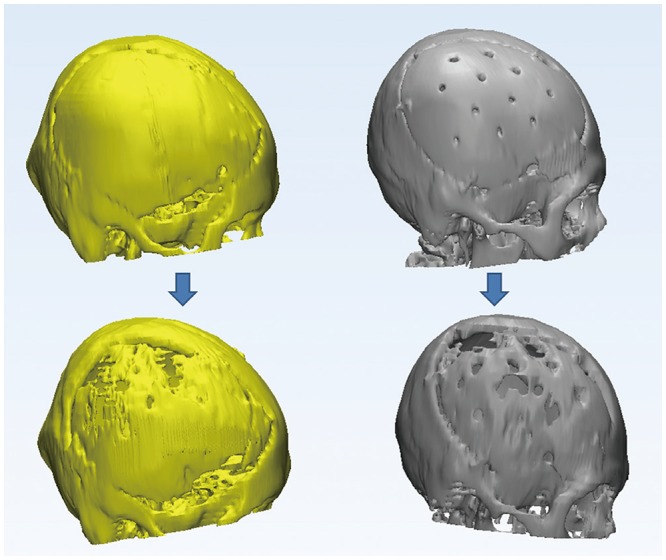
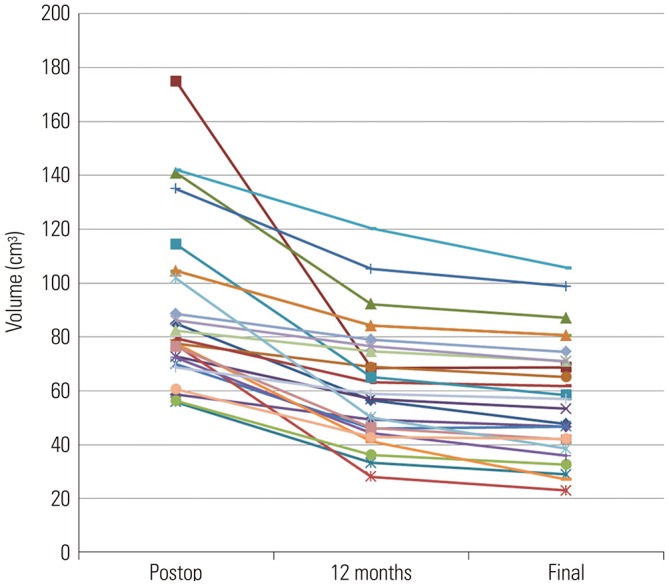
 XML Download
XML Download