Abstract
Morus alba L., known as white mulberry, is a medicinal plant belongs to family Moraceae. It has long been used commonly in Ayurvedic for the treatment of lung-heat, cough, asthma, hematemesis, dropsy and hypertension. In the present study, seven prenylated flavonoids, along with four benzofuran compounds were isolated by means of repeated column chromatography. The structures of the known compounds were identified as kuwanon G (1), kuwanon E (2), kuwanon T (3), morusin (4), sanggenon A (5), sanggenon M (6), sanggenol A (7), moracin R (8), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11), by comparing their spectroscopic data with those reported in the literature. For these isolates, containing trace compounds, the inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells was examined. All isolated compounds (1 - 11) showed excellent inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells. Especially this study is first time to report that sanggenon A (5), sanggenon M (6), sanggenol A (7), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11) showed the inhibitory activity of IL-6 production. Our study suggested the possibility of anti-inflammatory regulation by compounds (1 - 11) isolated from M. alba.
The root bark of Morus alba L., called “Sang-Baek-Pi” in Korea, has been used in traditional medicines for the treatment of lung-heat, cough, asthma, hematemesis, dropsy and hypertension.1 Previous phytochemical investigations resulted in the isolation of polyphenolic constituents including prenylated flavonoids, benzofurans and Diels-Alder type adducts with various biological activities such as cytotoxicity, antioxidant, cancer cell invasion, migration and hepatoprotection.234 In addition, the prenylated flavonoids, main constituents of this plant, have also been shown to exhibit various anti-inflammatory activities.56 However, the pharmacological evaluation on the minor constituents of the root bark of M. alba has not been performed in detail, because of its difficulty for the isolation and the presence of trace amount. In the present study, by means of repeated column chromatography using silica gel, Sephadex LH-20 and LiChroprep RP-18, seven prenylated flavonoids, along with four benzofuran compounds were isolated. The structures of the known compounds were identified as kuwanon G (1), kuwanon E (2), kuwanon T (3), morusin (4), sanggenon A (5), sanggenon M (6), sanggenol A (7), moracin R (8), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11), by comparing their spectroscopic data with those reported in the literature. For these isolates, containing trace compounds, the inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells was examined. This paper reports the isolation and structural characterization of these compounds and their inhibitory activities against IL-6 production.
IR spectra were recorded on an IMS 85 (Bruker). The EI-MS (70 eV) spectra were obtained on a JEOL JMS-AX 505H. NMR spectra, including NOESY, COSY, heteronuclear multiple quantum coherence (HMQC) and HMBC experiments, were recorded on a Varian UNITY INOVA 500 NMR spectrometer (KBSI-Gwangju center) operating at 500 MHz (1H) and 125MHz (13C), respectively, with chemical shifts given in ppm (δ). TLC was carried out on precoated Kieselgel 60 F254 (art. 5715, Merck) and RP-18 F254s (art. 15389, Merck) plates. Column chromatography was performed on silica gel 60 (40 - 63 and 63 - 200 µm, Merck), MCI gel CHP20P (75 - 150 µm, Mitsubishi Chemical Co.), and Sephadex LH-20 (25 - 100 µm, Sigma). Low pressure liquid chromatography was carried out over a Merck Lichroprep Lobar®-A RP-18 (240 × 10 mm) column with a FMI QSY-0 pump (ISCO).
The root bark of Morus alba L. were collected in Geongju, Geongbuk province, Korea, and authenticated by Prof. J. H. Lee (Dongguk University, Gyoungju, Korea). The voucher specimen (CSU-1048-17) was deposited in the Herbarium of the College of Pharmacy, Chosun University.
The dried root barks of M. alba (12 kg) were extracted three times with MeOH under reflux and 1511.6 g of residue were produced. The MeOH extract was suspended in water and then partitioned sequentially with equal volumes of dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol (n-BuOH). Each fractions were evaporated in vaccuo to yield the residues of CH2Cl2 (318.2 g), EtOAc (192.2 g), n-BuOH (182.4 g), and water (534.3 g) extract. The CH2Cl2 fraction (25.0 g) was chromatographed over a silica gel column using a gradient solvent system of n-hexane-EtOAc (10:1→1:5, MeOH) to give six subfractions (D1-D6). Subfraction D3 (3.1 g) was subjected to silica gel column chromatography (CC) eluting with a gradient solvent system of n-hex-EtOAc (5:1→1:1, MeOH) to yield eight subfractions (D31-D38). Subfraction D33 (0.88 g) was purified by repeated LiChroprep RP 18 CC (MeOH-H2O, 5:1) and prep-HPLC (75% aq. MeOH) to give 5 (56.20 mg) and 6 (24.10 mg), respectively. Subfraction D34 (0.41 g) was purified by repeated LiChroprep RP 18 CC (MeOH-H2O, 5:1) and prep-HPLC (78% aq. MeOH) to give 7 (14.3 mg). The EtOAc fraction (12.1 g) was chromatographed over a silica gel column using a gradient solvent system of n-hexane-EtOAc (2:1→1:8, EtOAc, MeOH) to give five subfractions (E1-E5). Subfraction E1 (0.6 g) was subjected to silica gel CC eluting with a gradient solvent system of n-hex-EtOAc (10:1→4:1, MeOH) to yield seven subfractions (E11-E17). Subfraction E15 (0.39 g) was purified by MCI gel CC (MeOH-H2O, 3:1) to give 3 (16.29 mg) and 4 (122.40 mg), respectively. Subfraction E17 (0.13 g) was purified by prep-HPLC (80% aq. MeOH) to yield 10 (4.50 mg) and 11 (11.80 mg), respectively. Subfraction E2 (3.88 g) was subjected to MCI gel column chromatography (CC) eluting with a gradient solvent system of MeOH-H2O (1:1 →3:1) to yield sixteen subfractions (E21-E216). Subfraction E23 (0.033 g) was purified by LiChroprep RP 18 CC (MeOH-H2O, 1:1) to yield 8 (5.22 mg). Subfraction E24 (0.212 g) was subjected to silica gel CC (CHCl3-MeOHH2O, 12:1:0.1→8:1:0.1) and LiChroprep RP 18 CC (MeOHH2O, 1:1) to yield 1 (0.18 g). Subfraction E27 (0.57 g) was purified repeated silica gel CC (CHCl3-MeOH-H2O, 25:1:0.1→10:1:0.1) and LiChroprep RP 18 CC (MeOHH2O, 2:1) to yield 2 (40.0mg) and 9 (5.61mg), respectively.
Pale yellowish amorphous powder; IR (KBr) νmax: 3350, 1650 cm-1; 1H-NMR (Acetone-d6, 300 MHz): δ 7.41 (1H, d, J = 8.0 Hz, H-6′ or H-27), 7.29 (1H, d, J = 8.0 Hz, H-6′ or H-27), 6.78 (1H, d, J = 8.0 Hz, H-33), 6.67 (1H, d, J = 2.0 Hz, H-3′), 6.55 (1H, dd, J = 2.0, 8.0 Hz, H-5′), 6.21 (1H, d, J = 2.0 Hz, H-30), 6.08 (1H, dd, J = 2.0, 8.0 Hz, H-32), 6.03 (1H, d, J = 2.0 Hz, H-24), 5.98 (1H, s, H-6), 5.93 (1H, dd, J = 2.0, 8.0 Hz, H-26), 4.95-5.40 (2H, m, H-10 and H-15), 4.30-4.70 (2H, m, H-14 and H-20), 3.30-3.90 (1H, m, H-19), 3.17 (2H, d, J = 7.0 Hz, H-9), 1.80-2.20 (1H, m, H-18), 1.62 (3H, s, H-12), 1.52 (3H, s, H-16), 1.48 (3H, s, H-13); 13C-NMR (Acetone-d6, 75 MHz): δ 208.1 (C-21), 181.7 (C-4), 164.2 (C-23), 164.2 (C-25), 161.3 (C-7), 160.83 (C-4′), 160.3 (C-8a), 159.2 (C-2), 156.3 (C-2′), 155.8 (C-29), 155.8 (C-31), 155.2 (C-5), 132.8 (C-16), 132.4 (C-33), 131.2 (C-11), 131.2 (C-6′), 130.8 (C-27), 123.2 (C-15), 121.8 (C-10), 120.7 (C-28), 119.7 (C-3), 114.0 (C-22), 111.4 (C-1′), 107.2 (C-26), 106.7 (C-8), 106.7 (C-32), 106.7 (C-5′), 103.7 (C-4a), 102.6 (C-24), 102.6 (C-3′), 101.9 (C-30), 97.5 (C-6), 45.8 (C-20), 38.3 (C-18), 38.3 (C-19), 25.4 (C-12), 23.5 (C-9), 22.9 (C-14), 22.5 (C-17), 17.3 (C-13); EI-MS m/z 629 [M]+.
Pale yellowish amorphous powder; IR (Nujol) νmax: 3360, 1650, 1595 cm-1; 1H-NMR (Acetone-d6, 600 MHz): δ 7.20 (1H, s, H-6′), 6.50 (1H, s, H-3′),5.95 (1H, d, J = 1.8 Hz, H-6), 5.94 (1H, d, J = 1.8 Hz, H-8), 5.70 (1H, dd, J = 3.0, 13.8 Hz, H-2), 5.35 (1H, dt, J = 1.2, 7.2 Hz, H-2″), 5.11 (1H, tt, J = 1.2, 7.2 Hz, H-7″), 3.27 (2H, d, J = 7.2 Hz, H-1″), 3.20 (1H, dd, J = 13.8, 17.4 Hz, H-3a), 2.69 (1H, dd, J = 3.0, 17.4 Hz, H-3b), 2.09 (2H, m, H-6″), 2.02 (2H, t, J = 7.2 Hz, H-5″), 1.71 (3H, s, H-4″), 1.62 (3H, s, H-9″), 1.57 (3H, s, H-10″); 13C-NMR (Acetone-d6, 150 MHz): δ 197.8 (C-4), 167.3 (C-7), 165.4 (C-8a), 164.9 (C-5), 156.7 (C-4′), 154.2 (C-2′), 135.9 (C-3″), 131.7 (C-8″), 129.0 (C-6′), 125.2 (C-7″), 124.1 (C-2″), 120.2 (C-5′), 116.9 (C-1′), 103.4 (C-3′), 103.2 (C-4a), 96.7 (C-8), 95.9 (C-6), 75.5 (C-2), 42.7 (C-3), 40.5 (C-5″), 28.4 (C-1″), 27.5 (C-6″), 25.9 (C-9″), 17.8 (C-10″), 16.3 (C-4″); EI-MS m/z: 424 [M]+.
Pale yellowish amorphous powder; 1H-NMR (CD3OD, 300 MHz): δ 6.89 (1H, d, J = 8.4 Hz, H-6′), 6.44 (1H, d, J = 8.4 Hz, H-5′), 6.27 (1H, d, J = 2.2 Hz, H-8), 6.17 (1H, d, J = 2.2 Hz, H-6), 5.25 (1H, br t, J = 5.9 Hz, H-15), 5.08 (1H, br t, J = 5.9 Hz, H-10), 3.33 (2H, d, J = 6.9 Hz, H-9a, 14a), 3.08 (2H, d, J = 6.9 Hz, H-9b, 14b), 1.78 (3H, s, CH3-17), 1.67 (3H, s, CH3-18), 1.58 (3H, s, CH3-12), 1.33 (3H, s, CH3-13); 13C-NMR (CD3OD, 75 MHz): δ 183.9 (C-4), 165.7 (C-7), 163.8 (C-2), 163.3 (C-5), 160.1 (C-8a), 159.4 (C-4′), 154.9 (C-2′), 132.9 (C-16), 132.0 (C-11), 128.9 (C-6′), 124.1 (C-15), 122.7 (C-10), 122.2 (C-3), 117.9 (C-3′), 114.0 (C-1′), 108.3 (C-5′), 105.6 (C-4a), 99.6 (C-6), 94.7 (C-8), 26.1 (C-17), 26.0 (C-12), 25.0 (C-9), 23.5 (C-14), 18.2 (C-18), 17.8 (C-13).
Pale yellowish amorphous powder; 1HNMR (CD3OD, 500 MHz): δ 7.11 (1H, d, J = 8.3 Hz, H-6′), 6.58 (1H, d, J = 10.0 Hz, H-14), 6.42 (1H, d, J = 2.0 Hz, H-3′), 6.41 (1H, dd, J = 2.0, 8.3 Hz, H-5′), 6.14 (1H, s, H-6), 5.56 (1H, d, J = 10.0 Hz, H-15), 5.09 (2H, t, J = 6.7 Hz, H-10), 3.10 (2H, d, J = 6.9 Hz, H-9), 1.58 (3H, s, CH3-12), 1.42 (6H, s, CH3-17, 18), 1.40 (3H, s, CH3-13); 13C-NMR (CD3OD, 125 MHz): δ 184.1 (C-4), 163.7 (C-7), 162.8 (C-4′), 162.1 (C-2), 160.6 (C-8a), 158.1 (C-2′), 154.9 (C-5), 133.0 (C-11), 132.6 (C-6′), 128.3 (C-15), 122.8 (C-10), 122.2 (C-3), 115.9 (C-14), 113.3 (C-1′), 108.2 (C-5′), 106.1 (C-8), 104.0 (C-3′), 102.4 (C-4a), 100.3 (C-6), 79.3 (C-16), 28.6 (C-17), 28.6 (C-18), 26.0 (C-12), 25.0 (C-9), 17.8 (C-13).
Pale yellowish amorphous powder; 1H-NMR (CD3OD, 500 MHz): δ 7.25 (1H, d, J = 8.2 Hz, H-6′), 6.57 (1H, d, J = 10.1 Hz, H-14), 6.46 (1H, dd, J = 2.1, 8.2 Hz, H-5′), 6.34 (1H, d, J = 2.1 Hz, H-2′), 5.72 (1H, s, H-8), 5.56 (1H, d, J = 10.1 Hz, H-15), 5.18 (1H, t, J = 7.3 Hz, H-10), 3.10 (1H, dd, J = 9.1, 14.4 Hz, H-9a), 2.73 (1H, dd, J = 6.2, 14.4 Hz, H-9b), 1.61 (3H, s, CH3-12), 1.49 (3H, s, CH3-13), 1.40 (3H, s, CH3-17), 1.39 (3H, s, CH3-18); 13C-NMR (CD3OD, 125 MHz): δ 189.7 (C-4), 164.7 (C-7), 163.8 (C-8a), 161.8 (C-2′), 161.8 (C-4′), 161.7 (C-5), 137.5 (C-11), 127.6 (C-15), 125.8 (C-6′), 121.4 (C-1′), 119.1 (C-10), 116.0 (C-14), 110.1 (C-5′), 99.8 (C-3′), 99.8 (C-4a), 96.8 (C-8), 79.8 (C-16), 32.6 (C-9), 28.8 (C-18), 28.7 (C-17) 26.1 (C-12), 18.3 (C-13).
Pale yellowish amorphous powder; 1H-NMR (CD3OD, 500 MHz): δ 7.30 (1H, d, J = 8.2 Hz, H-6′), 6.46 (1H, dd, J = 2.1, 8.2 Hz, H-5′), 6.45 (1H, d, J = 10.1 Hz, H-14), 6.34 (1H, d, J = 2.1 Hz, H-2′), 5.85 (1H, s, H-6), 5.54 (1H, d, J = 10.1 Hz, H-15), 5.20 (1H, t, J = 7.3 Hz, H-10), 3.12 (1H, dd, J = 9.1, 14.4 Hz, H-9a), 2.75 (1H, dd, J = 6.2, 14.4 Hz, H-9b), 1.58 (3H, s, CH3-12), 1.52 (3H, s, CH3-13), 1.42 (3H, s, CH3-17), 1.38 (3H, s, CH3-18); 13C-NMR (CD3OD, 125 MHz): δ 189.7 (C-4), 164.9 (C-7), 161.9 (C-4′), 161.9 (C-2′), 161.7 (C-5), 158.0 (C-8a), 137.8 (C-11), 127.6 (C-15), 125.8 (C-6′), 121.5 (C-1′), 118.9 (C-10), 116.5 (C-14), 110.2 (C-5′), 101.4 (C-4a), 99.8 (C-3′), 97.8 (C-8), 79.7 (C-16), 32.7 (C-9), 28.7 (C-18), 28.7 (C-17) 26.2 (C-12), 18.4 (C-13).
Pale yellowish amorphous powder; 1H-NMR (CD3OD, 300 MHz): δ 7.08 (1H, d, J = 8.4 Hz, H-6′), 6.43 (1H, d, J = 8.4 Hz, H-5′), 5.91 (1H, d, J = 1.8 Hz, H-6), 5.88 (1H, d, J = 1.8 Hz, H-8), 5.65 (1H, dd, J = 2.6, 12.8 Hz, H-2), 5.21 (1H, t, J = 7.0 Hz, H-2″), 5.07 (1H, t, J = 7.3 Hz, H-7″), 3.35 (2H, d, J = 6.2 Hz, H-1″), 3.10 (1H, dd, J = 17.2, 13.2 Hz, H-3a), 3.10 (1H, dd, J = 17.2, 2.9 Hz, H-3b), 2.03 (2H, m, H-6″), 1.97 (2H, m, H-5″), 1.77 (3H, s, H-4″), 1.62 (3H, s, H-9″), 1.56 (3H, s, H-10″); 13C-NMR (CD3OD, 75 MHz): δ 198.5 (C-4), 168.8 (C-7), 165.6 (C-8a), 165.3 (C-5), 157.6 (C-4′), 154.2 (C-2′), 136.2 (C-3″), 132.3 (C-8″), 125.7 (C-6′), 125.5 (C-7″), 124.1 (C-2″), 119.0 (C-3′), 117.6 (C-1′), 108.6 (C-5′), 103.4 (C-4a), 97.3 (C-6), 96.5 (C-8), 76.8 (C-2), 43.3 (C-3), 41.1 (C-5″), 27.8 (C-6″), 26.0 (C-10″), 23.4 (C-1″), 17.9 (C-9″), 16.5 (C-4″).
Pale yellowish amorphous powder; 1H-NMR (Acetone-d6, 600 MHz): δ 7.25 (1H, s, H-4), 7.00 (1H, d, J = 1.2 Hz, H-3), 6.87 (1H, s, H-7), 6.86 (2H, d, J = 2.4 Hz, H-2′, H-6′), 6.37 (1H, t, J = 2.4 Hz, H-4′), 3.81 (1H, dd, J = 5.4, 8.4 Hz, H-2″), 3.10 (1H, dd, J = 5.4, 16.8 Hz, H-1″a), 2.82 (1H, ddd, J = 1.2, 8.4, 16.8 Hz, H-1″b), 1.37 (3H, s, CH3-5″), 1.25 (3H, s, CH3-4″); 13C-NMR (Acetone-d6, 150 MHz): δ 159.8 (C-3′), 159.8 (C-5′), 155.8 (C-2), 155.4 (C-7a), 152.4 (C-6), 133.3 (C-1′), 123.5 (C-3a), 121.7 (C-4), 117.9 (C-5), 103.7 (C-2′), 103.7 (C-6′), 103.5 (C-4′), 101.9 (C-3), 99.4 (C-7), 78.1 (C-3″), 69.9 (C-2″), 32.4 (C-1″), 26.3 (C-4″), 20.6 (C-5″).
Pale yellowish amorphous powder; 1H-NMR (Acetone-d6, 600 MHz): δ 7.41 (1H, d, J = 8.4 Hz, H-4), 7.24 (1H, d, J = 8.4 Hz, H-14″), 7.14 (1H, d, J = 8.4 Hz, H-20″), 7.05 (1H, s, H-3), 6.98 (1H, d, J = 1.8 Hz, H-2′), 6.97 (1H, d, J = 2.4 Hz, H-7), 6.94 (1H, d, J = 1.8 Hz, H-6′), 6.81 (1H, dd, J = 2.4, 8.4 Hz, H-5), 6.50 (1H, dd, J = 2.4, 8.4 Hz, H-19″), 6.45 (1H, d, J = 5.4 Hz, H-2″), 6.43 (1H, d, J = 2.4 Hz, H-17″), 6.38 (1H, d, J = 2.4 Hz, H-11″), 6.23 (1H, dd, J = 2.4, 8.4 Hz, H-13″), 3.49 (1H, s, H-5″), 3.36 (1H, dd, J = 5.4, 12.0 Hz, H-3″), 2.98 (1H, ddd, J = 5.4, 11.4, 11.4 Hz, H-4″), 2.73 (1H, dd, J = 5.4, 17.4 Hz, H-6″a), 2.05 (1H, dd, J = 5.4, 17.4 Hz, H-6″b), 1.77 (3H, s, CH3-7″); 13C-NMR (Acetone-d6, 150 MHz): δ 159.9 (C-12″), 157.9 (C-10″), 157.7 (C-7a), 157.5 (C-3′), 156.7 (C-2), 156.7 (C-5′), 155.0 (C-6), 154.6 (C-18″), 153.4 (C-16″), 133.8 (C-1″), 131.1 (C-1′), 130.4 (C-14″), 127.9 (C-20″), 122.9 (C-2″), 122.5 (C-3a), 122.1 (C-4), 117.6 (C-4′), 116.9 (C-15″), 113.4 (C-5), 113.3 (C-9″), 109.8 (C-19″), 107.1 (C-13″), 105.3 (C-6′), 105.1 (C-2′), 104.5 (C-17″), 103.9 (C-11″), 102.6 (C-8″) 102.3 (C-3), 98.4 (C-7), 37.2 (C-3″), 36.3 (C-6″), 35.2 (C-5″), 28.5 (C-4″), 23.9 (C-7″).
Pale yellowish amorphous powder; 1H-NMR (CD3OD, 500 MHz): δ 7.34 (1H, d, J = 8.4 Hz, H-4), 6.89 (1H, d, J = 2.0 Hz, H-5′), 6.74 (1H, dd, J = 2.1, 8.4 Hz, H-5), 6.72 (1H, d, J = 2.4 Hz, H-6′), 6.69 (1H, s, H-3), 6.47 (1H, t, J = 2.3 Hz, H-4′), 5.08 (1H, t, J = 7.2 Hz, H-2″), 5.05 (1H, t, J = 7.2 Hz, H-7″), 3.82 (3H, s, -OCH3), 3.44 (2H, d, J = 6.2 Hz, H-1″), 2.03 (2H, m, H-6″), 1.95 (2H, m, H-5″), 1.65 (3H, s, H-4″), 1.61 (3H, s, H-9″), 1.55 (3H, s, H-10″); 13C-NMR (CD3OD, 125 MHz): δ 160.5 (C-3′), 157.5 (C-7a), 157.2 (C-5′), 156.8 (C-6), 156.0 (C-2), 135.3 (C-3″), 133.1 (C-1′), 132.2 (C-8″), 125.8 (C-2″), 125.6 (C-7″), 123.1 (C-3a), 122.1 (C-4), 120.9 (C-2′), 113.2 (C-5), 108.3 (C-6′), 106.0 (C-3), 100.4 (C-4′), 98.6 (C-7), 56.3 (-OCH3), 40.9 (C-5″), 27.8 (C-6″), 26.6 (C-1″), 26.0 (C-10″), 17.8 (C-9″), 16.6 (C-4″).
Pale yellowish amorphous powder; 1H-NMR (CD3OD, 500 MHz): δ 7.32 (1H, d, J = 8.5 Hz, H-4), 6.93 (1H, s, H-3), 6.92 (1H, d, J = 8.5 Hz, H-5), 6.81 (2H, d, J = 2.2 Hz, H-2′, H-6′), 6.26 (1H, t, J = 2.2 Hz, H-4′), 5.35 (1H, t, J = 7.4 Hz, H-2″), 4.99 (1H, t, J = 7.4 Hz, H-7″), 3.87 (3H, s, -OCH3), 3.61 (2H, d, J = 7.4 Hz, H-1″), 2.05 (2H, m, H-6″), 1.97 (2H, m, H-5″), 1.87 (3H, s, H-4″), 1.55 (3H, s, H-10″), 1.51 (3H, s, H-9″); 13C-NMR (CD3OD, 125 MHz): δ 160.1 (C-3′, C-5′), 156.8 (C-2), 156.5 (C-6), 155.5 (C-7a), 136.2 (C-3″), 134.0 (C-1′), 132.3 (C-8″), 125.4 (C-7″), 124.4 (C-3a), 123.6 (C-2″), 119.2 (C-4), 114.7 (C-7), 109.2 (C-5), 104.3 (C-2′, 6′), 103.8 (C-4′), 102.5 (C-3), 57.2 (-OCH3), 40.9 (C-5″), 27.7 (C-6″), 25.8 (C-9″), 23.7 (C-1″), 17.8 (C-10″), 16.6 (C-4″).
IL-6 bioassay was carried out using a slight modification of an established method.78 Briefly, 500 µL of the MG-63 cells (3 × 104 cells/mL) in DMEM containing 10% FBS were dispensed into a 24-well plate, the culture was incubated for 24 h at 37℃. Then, 5 µL of TNF-α (10 ng/mL), 5 µL of BAY 11-7085 (10 ng/mL), and 5 µL of the DMSO with or without the compounds (100 µg/mL) were added. After incubation at 37℃ with 5% CO2 for 24 h, the medium was stored at -20℃ until measurement. The IL-6 content of the medium was measured in an ELISA procedure. 96-well plates were coated with 100 µL of purified rat anti-human IL-6 monoclonal antibody in 0.1M NaHCO3 (pH 9.6) by overnight incubation at 4℃. The wells were blocked with 200 µL of 3% BSA in PBS for 2 h at room temperature (RT) and then incubated with 100 µL of specific antibody for 2 h at RT. 100 µL of HRP conjugated rabbit anti-goat IgG (1:1000 dilution) was added to each well and incubated for 2 h at RT. 100 mL of TMB (3,3',5,5'-tetramethyl-benzidine) substrate solution was added and incubated for 10 min at RT. The color reaction was stopped with 50 µL of 0.4 N HCl and the optical density was read at 450 nm using a Microplate Reader (Molecular Devices Co., Ltd., U.S.A.).
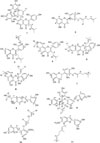
Repeated column chromatography of the CH2Cl2 and EtOAc soluble fractions of the root bark of M. alba yielded eleven compounds (1 - 11) (Fig. 1). The structures of eleven known compounds were identified as kuwanon G (1), kuwanon E (2), kuwanon T (3), morusin (4), sanggenon A (5), sanggenon M (6), sanggenol A (7), moracin R (8), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11), by comparing their spectroscopic data with those reported in the literature.349101112131415
Among 11 isolates, compounds 8 - 10 were minor compounds. IL-6 is a cytokine, originally identified as a T-cell derived factor that regulates B-cell growth and differentiation.16 Human IL-6 is an important component of the inflammatory cascade. Dysregulation of IL-6 production has been implicated in a variety of inflammatory/autoimmune disease states, including rheumatoid arthritis, cardiac myxoma, Castleman's disease, and mesangial proliferative glomerulonephritis.17 The proinflammatory cytokines IL-1 and TNF-α markedly stimulate the production IL-6.18
The inhibitory activity of the isolated compounds (1 - 11) against IL-6 production in TNF-α stimulated MG-63 cells was examined. In our results, all isolated compounds (1 - 11) showed excellent inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells (Table 1). In the previous other studies, it has already reported that M. alba has an anti-inflammatory effect. In addition, other previous studies also reported that some components from M. alba have regulated the proinflammatory cytokines.61920 Especially, among the 11 compounds isolated from M. alba in this study, some compounds already have been reported to regulate inflammatory factors in this previous study.
First, in the previous studies, kuwanon G (1) isolated from M. alba significantly decreased inflammatory cytokines in macrophages,21 and inhibited the IgE and Th2 cytokines including IL-4, IL-5, and IL-13 productions in a mouse model of asthma.22 In addition, it was already reported that kuwanon E (2), kuwanon T (3), and moracin R isolated from M. alba showed significant reduction of nitric oxide (NO) production in RAW264.7 cells.6 Morusin is a well-known to strongly inhibit the inflammatory action and it is also reported particularly effective in the regulation of cytokines.62324 Similar to previous other studies, our present study also demonstrated that kuwanon G (1), kuwanon E (2), kuwanon T (3), morusin (4), and moracin R (8) significantly inhibited IL-6 production in MG-63 cells (Table 1).
However, the inhibitory effect of cytokine production or inflammatory response by sanggenon A (5), sanggenon M (6), sanggenol A (7), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11) has not been reported yet. Sanggenon C and O have been reported to inhibit the inflammatory response through inhibition of NF-κB activation,25 and sanggenon B and sanggenon D are known to regulate cyclooxygenases and lipoxygenases.23 However sanggenon A (5) and sanggenon M (6) were not known about the anti-inflammatory effects. In addition, sanggenol A (7) was reported to protective effects on glutamate-induced neuronal cell death,26 but the regulating effects of anti-inflammatory and cytokine were not noted. Furthermore, among mulberofuran type compounds, only mulberofuran K has been reported to have anti-inflammatory activity,27 but mulberofuran G (9), mulberofuran A (10), and mulberofuran B (11) have unknown to the regulatory action of inflammatory cytokines. In the previous studies, mulberofuran G (9) only showed antioxidative action,28 strong antibacterial activity,29 and anti-hepatitis B virus activity.30 Therefore, this study is first time to report that sanggenon A (5), sanggenon M (6), sanggenol A (7), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11) isolated from M. alba showed the inhibitory activity of IL-6 production, and it suggested the possibility of anti-inflammatory regulation by these isolated compounds (1 - 11). Further research will be conducted to investigate the mechanism of anti-inflammatory activity or IL-6 inhibition by the isolated compounds (1 - 11).
Figures and Tables
Table 1
Inhibitory effect of compounds 1 - 11 against IL-6 production in TNF-α stimulated MG 63 cells.a

aMG-63 cells (3×104 cell/well) were incubated for 24 h. Cultures were incubated with or without compounds (100 µg/mL) for 30 min and then stimulated with TNF-α (10 ng/mL) for 24 h. IL-6 in the supernatant was measured by ELISA as described in Materials and Methods. Results are expressed as the mean ± S.E. from three different experiments. BAY 11-7085 was used as a positive control. *P < 0.05 or **P < 0.01 compared with TNF-α treated value.
References
1. Bae KH. The Medicinal Plants of Korea. Seoul: Kyo Hak Pub. Co.;2000. p. 73.
2. Hano Y, Hirakura K, Nomura T, Terada S, Fukushima K. Planta Med. 1984; 50:127–130.
3. Nomura T, Fukai T, Narita T. Heterocycle. 1980; 14:1943–1951.
4. Nomura T, Fukai T. Planta Med. 1981; 42:79–88.
5. Nomura T. Yakugaku Zassh. 2001; 121:535–556.
6. Lim HJ, Jin HG, Woo ER, Lee SK, Kim HP. J Ethnopharmacol. 2013; 149:169–175.
7. Kim BH, Chung EY, Ryu JC, Jung SH, Min KR, Kim Y. Arch Pharm Res. 2003; 26:306–311.
8. Liu QH, Jeong JE, Choi EJ, Moon YH, Woo ER. Arch Pharm Res. 2006; 29:1109–1113.
9. Chung MI, Lu CM, Huang PL, Lin CN. Phytochemistry. 1995; 40:1279–1282.
10. Du J, He ZDm, Jiang RW, Ye WC, Xu HX, But PP. Phytochemistry. 2003; 62:1235–1238.
11. Shen RC, Lin M. Phytochemistry. 2001; 57:1231–1235.
12. Tan YX, Liu C, Zhang T, Chen RY, Yu DQ. Phytochem Lett. 2010; 3:57–61.
13. Kapche GD, Fozing CD, Donfack JH, Fotso GW, Amadou D, Tchana AN, Bezabih M, Moundipa PF, Ngadjui BT, Abegaz BM. Phytochemistry. 2009; 70:216–221.
14. Hano Y, Fukai T, Nomura T, Uzawa J, Fukushima K. Chem Pharm Bull. 1984; 32:1260–1263.
15. Nomura T, Fukai T. Planta Med. 1981; 42:197–199.
16. Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, Tsunsawa S, Sakiyama F, Matsui H, Takahara Y, Taniguchi T, Kishimoto T. Nature. 1986; 324:73–76.
17. Hirano T, Akira S, Taga T, Kishimoto T. Immunol Today. 1990; 11:443–449.
18. Van Damme J, Opdenakker G, Simpson RJ, Rubira MR, Cayphas S, Vink A, Billiau A, Van Snick J. J Exp Med. 1987; 165:914–919.
19. Qin J, Fan M, He J, Wu XD, Peng LY, Su J, Cheng X, Li Y, Kong LM, Li RT, Zhao QS. Nat Prod Res. 2015; 29:1711–1718.
20. Wu YX, Kim YJ, Kwon TH, Tan CP, Son KH, Kim T. Nat Prod Res. 2018; 23:1–4.
21. Liu XX, Zhang XW, Wang K, Wang XY, Ma WL, Cao W, Mo D, Sun Y, Li XQ. Toxicol Appl Pharmacol. 2018; 341:56–63.
22. Jung HW, Kang SY, Kang JS, Kim AR, Woo ER, Park YK. Phytother Res. 2014; 28:1713–1719.
23. Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP. Biochem Pharmacol. 2001; 62:1185–1191.
24. Tseng TH, Lin WL, Chang CK, Lee KC, Tung SY, Kuo HC. Cell Physiol Biochem. 2018; 51:1376–1388.
25. Dat NT, Binh PT, Quynh LTP, Huong HT, Minh CV. . Immunopharmacol Immunotoxicol. 2012; 34:84–88.
26. Jung JW, Ko WM, Park JH, Seo KH, Oh EJ, Lee DY, Lee DS, Kim YC, Lim DW, Han D, Baek NI. Arch Pharm Res. 2015; 38:2066–2075.
27. Shim SY, Sung SH, Lee M. Int Immunopharmacol. 2018; 58:117–124.
28. Kang J, Chen RY, Yu DQ. Planta Med. 2006; 72:52–59.
29. Sohn HY, Son KH, Kwon CS, Kwon GS, Kang SS. Phytomedicine. 2004; 11:666–672.
30. Geng CA, Ma YB, Zhang XM, Yao SY, Xue DQm. J Agric Food Chem. 2012; 60:8197–8202.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download