Abstract
The rhizomes of Dioscorea japonica Thunb. are widely consumed as food and also used to treat diabetes and polyuria in Korea. This study was undertaken to study the anti-atopic dermatitis effects of a 95% ethanolic extract (DJE) of D. japonica in an oxazolone-stimulated murine model of atopic dermatitis (AD). The therapeutic effects of DJE on AD-like skin lesions were assessed on both ears. DJE (1%) or dexamethasone (0.5%; the positive control) were applied to skin lesions for three weeks. Serum levels of IgE and IL-4 were assessed by ELISA (enzyme-linked immunosorbent assay). Histopathological examinations were performed by hematoxylin and eosin (H&E) and toluidine blue staining and revealed DJE significantly reduced dermal thickness and inflammatory cell infiltration when applied to oxazolone-treated ear skin. DJE-treated AD mice also showed lower serum levels of IgE and IL-4 than oxazolone-stimulated controls. Our findings demonstrate DJE might be a useful safe, topical agent for the treatment of atopic diseases.
Atopic dermatitis (AD) is a chronically relapsing inflammatory skin disease that often precedes allergic disorders.1 AD has a prevalence of 10–20% in children and of 1–3% in adults.2 It is characterized by clinical symptoms like severe itching, rash, edema, hemorrhage, and erosion.3 T helper 2 (TH2) cells and TH2-type cytokines (IL-4, IL-5, and IL-13) play essential roles in AD.45 In particular, increased IL-4 production in AD promotes IgE hyperproduction, which is a major feature of atopic diseases.6 Pimecrolimus (Elidel, SDZ ASM 981) is a cell-selective inhibitor of inflammatory cytokines and was specifically developed for the treatment of inflammatory skin diseases such as AD, allergic contact dermatitis, and irritant contact dermatitis.7 Pimecrolimus blocks the productions of inflammatory cytokines, such as TH1 (IL-2, IFN-γ) and TH2 (IL-4, IL-10) cytokines, in T cells and mast cells and has proven to be an effective short- and long-term therapy for AD.78
Dioscorea japonica Thunb., also known as ‘San Yak’, is a member of the Dioscoreaceae family and is a folk medicine used to treat syndromes related to metabolic disorders including diabetes in Korea.9 Plants of the Dioscorea genus have been reported to possess diverse pharmacological activities, which include anti-inflammatory, anti-tumor, and immunomodulatory effects.101112 The rhizomes of D. japonica are used to treat fatigue and reinforce stomach function, reduce chronic diarrhea, and moisturize skin in Chinese herbal medicine,13 and contain as secondary metabolites, steroidal saponins and sapogenins, including diosgenin, a starting material for the industrial production of steroidal drugs.1415 Although Dioscorea sp. have been extensively studied and reported to have many biological properties, limited information is available on the anti-AD activities of D. japonica extract. Accordingly, in the present study, we investigated the anti-atopic properties of an ethanolic extract of D. japonica using an oxazolone-induced murine model of AD.
Dried D. japonica rhizomes were purchased from Naturalmom (Namwon, Jeollabuk-Do, South Korea) and identified by Professor Eun Ju Jeong of the Department of Agronomy and Medicinal Plant Resources at Gyeongnam National University of Science and Technology (Jinju, Gyeongnam, South Korea). A voucher specimen (PNU-0025) was deposited at the Medicinal Herb Garden, Pusan National University. Dried D. japonica rhizomes (20 kg) was extracted with 95% EtOH (120 L) for 48 h, filtered, and the filtrate was freeze-dried to yield DJE (278.2 g).
Phytochemical constituents of DJE were analyzed using an Agilent 6530 Accurate-Mass Q-TOF LC/MS system (Agilent Technologies, Palo Alto, CA, USA). Dioscin and gracillin were isolated from DJE using silica gel column chromatography and used as standard marker compounds for phytochemical characterization of DJE. A bridged ethylsiloxane/silica hybrid (BEH) C18 column (2.1 × 100 mm, 1.7 µm, Waters, Milford, MA, USA) was used to analyze DJE sample at a flow rate of 0.45 mL/min using a 5 mM ammonium formate (solvent A) and acetonitrile (solvent B) gradient using the following conditions: 0 – 100% B (0 – 20 min); 100% B (21.1 – 25 min); 98% A (25.1 – 30.0 min). All MS acquisitions were performed using negative ionization mode. Mass spectra were recorded across the m/z range 100 to 1500 and the masses of all peaks were obtained at an accuracy of value.
BALB/c mice (female, six weeks old) were obtained from Orient Bio Inc. (Seongnam, South Korea) and maintained in an air-controlled room (25±5℃, 55 ± 5% RH). Standard laboratory chow and water were provided ad libitum. Mouse care and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals issued by the National Institute of Health (NIH publication No. 85-23, revised 1996) and were approved beforehand by the Institutional Animal Care and Use Committee of Korea Institute of Science and Technology (Certification No. KIST-2016-011).
To induce AD-like lesions on BALB/c mouse ears, 20 µl of 1% oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one), dissolved in propylene glycol/EtOH (7:3, v/v) was applied to mouse ears (D0). A week later, 0.1% oxazolone (20 µL) was applied to both ears at 2-day intervals for 3 weeks (D7–27), and over the same 3-week period 1% DJE (20 µl) or 0.5% dexamethasone (20 µl) were administered daily to animals in treatment groups; when applied on same days DJE or dexamethasone was administered 4 hours before oxazolone. Mice were divided into four groups: (1) the CON group (vehicle treatment), (2) the oxazolone group (0.1% oxazolone treatment), (3) the oxazolone-dexa group (0.1% oxazolone and 0.5% dexamethasone treatment), and (4) the oxazolone-DJE group (0.1% oxazolone and 1% DJR treatment). Normal control mice (CON) were administered a propylene glycol/EtOH mixture (7:3) and positive control mice (the oxazolone-dexa group) were administered 0.1% oxazolone and 0.5% dexamethasone. On D28 mice were sacrificed, and blood was collected for serum IgE and IL-4 analysis, and a histopathological examination was carried out.
Ear skins from BALB/c mice were fixed in 10% formalin for 24 hours, paraffin-embedded, and sectioned (2 – 3 mm). Sections were stained with hematoxylin and eosin (H&E) or toluidine blue, and examined under a light microscope (Olympus CX31/BX51, Olympus Optical Co., Tokyo) and photographed (TE-2000U, Nikon Instruments Inc., Melville, USA).
AD mice induced by oxazolone were treated with 1% DJE in propylene glycol/EtOH solution (7:3) (20 µL, Oxazolone-DJE group), 0.5% dexamethasone in propylene glycol/EtOH solution (7:3) (20 µL, oxazolone-dexa group; the positive control), or 20 µL of propylene glycol/EtOH solution (7:3; the negative control (Oxazolone group). On day 28, mean ear thickness in the oxazolone group was 118% thicker than in the CON group. Dermal application of 1% DJE inhibited the development of oxazolone-induced AD-like skin lesions (Fig. 1b), and remarkably reducing swelling and erythematic intensity. Ear thickness was reduced by 21% after DJE co-treatment as compared with the Oxazolone group (Fig. 1c). On the other hand, 0.5% dexamethasone totally inhibited oxazolone-induced increases in ear thickness.
To further investigate the effects of DJE on mouse ear skin, we first examined the effect of oxazolone on epidermal thickness and mast cells infiltration. H&E staining revealed skin hyperproliferation and inflammatory cell infiltration (Fig. 2a), and co-treatment with DJE significantly inhibited oxazolone-induced ear swelling by cell hyperplasia and lymphocytes infiltration in epidermis, but its effects were less pronounced than those of dexamethasone. DJE reduced oxazolone-induced epidermal hyperplasia by 44% in AD BALB/c mice ears (Fig. 2b). In addition, toluidine blue staining of mice ear skin showed that repeated application of oxazolone induced mast cell degranulation (Fig. 3a). Furthermore, numbers of degranulated mast cells in the oxazolone-DJE and oxazolone-dexa groups were significantly lower than that of the oxazolone group. Co-treatment with 1% DJE or 0.5% dexamethasone prevented oxazolone-induced mast cell infiltrations by 50% and 100%, respectively (Fig. 3b).
Dermal administration of DJE improved atopic clinical symptoms, and this was supported by reductions in the serum levels of IgE and IL-4 as compared with oxazolone-induced AD BALB/c mice. Increased serum IgE and IL-4 are major features of atopic disease. On D28, sensitization with oxazolone induced 3.5- and 2.2-fold increases in total IgE and IL-4 concentrations, respectively (Fig. 4). Co-treatment with 1% DJE resulted in a 67% decrease in serum IgE concentration versus the oxazolone group (Fig. 4a), and a 63% reduction in serum IL-4 level (Fig. 4b). Dexamethasone decreased serum IgE level by 89% (Fig. 4a) and serum IL-4 level by 100% (Fig. 4b) versus mice in the oxazolone group.
Phytochemical analysis of DJE and spiking samples with authentic standards of dioscin (1) and gracillin (2) and comparing UV spectra and retention times confirmed the presence of these two saponins in DJE. 1: dioscin (m/z 868.08 at tR 18.647 min) and 2: gracillin (m/z 884.08 at tR 19.676 min) (Fig. 5), which were present in DJE at concentrations of 386 mg/g and 165 mg/g, respectively.
Plant-derived extracts and phytochemicals are a rich source of medicinal agents for the prevention and treatment of allergic and inflammatory diseases.1617 Well-known phytochemicals, such as flavonoids, alkaloids, and saponins, with strong anti-inflammatory activities are often used to treat dermatitis, AD, eczema, psoriasis, and other inflammatory diseases of the skin.181920 Furthermore, these naturally-occurring compounds present low risks of adverse effects, and thus, provide effective alternative treatments for AD.
Chemical reagent (haptens; picryl chloride, trinitrochlorobenzene, 2,4-dinitrochlorobenzene or oxazolone)-induced murine models of contact dermatitis have contributed greatly to the study of human atopic dermatitis.21 Multiple oxazolone challenges have been reported to produce a chronic allergic contact dermatitis resulting from a TH2-like hypersensitivity reaction in mice.22 In the present study, repeated sensitization of both ears of BALB/c mice with oxazolone at 1% and then 0.1% produced significant epidermal hyperplasia and inflammatory cell infiltration at treated sites. We found these AD-like skin symptoms were remarkably suppressed by treatment with DJE for 21 days.
AD is associated with TH1-type response characterized by increased levels of interferon γ (IFN-γ) and interleukin 2 (IL-2), and TH2-type response characterized by increased levels of TH2-associated cytokines (IL-4, IL-5, and IL-13).2324 Our results indicated that DJE markedly reduced serum IgE and IL-4 levels in oxazolone-sensitized AD mice, and thus, suggested it might be useful for the ethnopharmacological treatment of Th2-mediated or allergic inflammation.
Phytochemical analysis of DJE demonstrated dioscin (385 mg/g) and gracillin (164 mg/g) (both saponins) were major components. The main biological and pharmacological properties of steroidal saponins in Dioscorea sp. are considered to be due to cytotoxic and antifungal activities.14 In recent years, dioscin has reached more attention as a promising multi-target candidate to treat various diseases.25 Many works have indicated that dioscin has potent anti-inflammation, immunoregulation, and anti-allergic effects through regulating multiple targets and biological processes.2627 Furthermore, in a previous study, we found gracillin improved contact dermatitis by reducing IgE production in and IL-4 by TH2 cells,28 which suggests gracillin may have been responsible for the anti-atopic effect of DJE.
Summarizing, DJE (a 95% EtOH extract of D. japonica rhizomes) suppressed erythema and edema of ear skin in our oxazolone-induced mouse model. Histopathological observations showed that DJE reduced inflammatory cell infiltration and epidermal hyperplasia in this model. Based on our observations, we suggest the described extract of D. japonica suppressed the development of AD-like skin lesions by inhibiting the up-regulations of IgE and IL-4 and that it be considered a potential anti-atopic agent.
Figures and Tables
 | Fig. 1Effects of the 95% ethanolic extract of Dioscorea japonica rhizomes (DJE) on the development of oxazolone-induced AD-like skin lesions on the ears of BALB/c mice. (a) Schematic of the experiment; (b) clinical features of oxazolone-induced AD-like skin symptoms; (c) changes in ear thicknesses. Results are presented as the means ± standard errors (n = 7) of two independent experiments. #P < 0.05 vs. the CON group; *P < 0.05 vs. the oxazolone group. CON: vehicle control group, oxazolone: oxazolone-treated group, oxazolone/dexa: oxazolone and 0.5% dexamethasone-treated group, oxazolone/DJE: oxazolone and 1% DJE-treated group. |
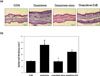 | Fig. 2Effects of DJE on histopathological change and epidermal thicknesses in oxazolone-sensitized BALB/c mice ears. (a) Hematoxylin and eosin (H&E) staining; (b) epidermal thicknesses. Results are presented as the means ± standard errors (n = 7) of two independent experiments. #P < 0.05 vs. the CON group; *P < 0.05 vs. the oxazolone group. DJE: 95% ethanolic extract of Dioscorea japonica rhizomes, CON: vehicle control group, oxazolone: oxazolone-treated group, oxazolone-dexa: oxazolone and 0.5% dexamethasone-treated group, oxazolone-DJE: oxazolone and 1% DJE-treated group. |
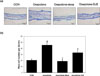 | Fig. 3Effects of DJE on oxazolone-induced histopathological changes and mast cell numbers in mouse ear skin. (a) toluidine blue staining; (b) mast cell numbers. Results are presented as the means ± standard errors (n = 7) of two independent experiments. #P < 0.05 vs. the CON group; *P < 0.05 vs. the oxazolone group. DJE: 95% ethanolic extract of Dioscorea japonica rhizomes, CON: vehicle control group, oxazolone: oxazolone-treated group, oxazolone-dexa: oxazolone and 0.5% dexamethasone-treated group, oxazolone-DJE: oxazolone and 1% DJE-treated group. |
 | Fig. 4Effects of DJE on total serum IgE and IL-4 levels in oxazolone-sensitized BALB/c mice ears. (a) total serum IgE concentration; (b) total serum IL-4 concentration. Results are presented as the means ± standard errors (n = 7) of two independent experiments. #P < 0.05 vs. the CON group; *P < 0.05 vs. the oxazolone group. DJE: 95% ethanolic extract of Dioscorea japonica rhizomes, CON: vehicle control group, oxazolone: oxazolone-treated group, oxazolone-dexa: oxazolone and 0.5% dexamethasone-treated group, oxazolone-DJE: oxazolone and 1% DJE-treated group. |
Acknowledgements
This research was supported by the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01282301)” Rural Development Administration, Republic of Korea.
References
1. Boguniewicz M, Leung DY. Immunol Rev. 2011; 242:233–246.
2. Larsen FS, Hanifin JM. Immunol Allergy Clin North Am. 2002; 22:1–24.
3. Rhodes AR. Clin Rev Allergy. 1986; 4:87–99.
4. Grewe M, Walther S, Gyufko K, Czech W, Schöpf E, Krutmann J. J Invest Dermatol. 1995; 105:407–410.
5. Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. Immunol Today. 1998; 19:359–361.
6. Jujo K, Renz H, Abe J, Gelfand EW, Leung DY. J Allergy Clin Immunol. 1992; 90:323–331.
7. Gupta AK, Chow M. J Eur Acad Dermatol Venereol. 2003; 17:493–503.
8. Stuetz A, Grassberger M, Meingassner JG. 1. Semin Cutan Med Surg. 2001; 20:233–241.
9. Kim MW. Korean J Nutr. 1998; 31:1377–1384.
10. Kim MJ, Kim HN, Kang KS, Baek NI, Kim DK, Kim YS, Jeon BH, Kim SH. Int Immunopharmacol. 2004; 4:1489–1497.
11. Hu K, Yao X. Cancer Invest. 2003; 21:389–393.
12. Zhao G, Kan J, Li Z, Chen Z. Carbohydr Polym. 2005; 61:125–131.
13. Wu JN. An illustrated Chinese Materia Medica. New York: Oxford University Press;2005. p. 264.
14. Sautour M, Mitaine-Offer AC, Lacaille-Dubois MA. J Nat Med. 2007; 61:91–101.
15. Liu H, Chou GX, Wu T, Guo YL, Wang SC, Wang CH, Wang ZT. 1. J Nat Prod. 2009; 72:1964–1968.
16. Bellik Y, Hammoudi SM, Abdellah F, Iguer-Ouada M, Boukraâ L. Recent Pat Inflamm Allergy Drug Discov. 2012; 6:147–158.
17. Dawid-Pać R. Postepy Dermatol Alergol. 2013; 30:170–177.
18. Kawai M, Hirano T, Higa S, Arimitsu J, Maruta M, Kuwahara Y, Ohkawara T, Hagihara K, Yamadori T, Shima Y, Ogata A, Kawase I, Tanaka T. Allergol Int. 2007; 56:113–123.
19. Souto AL, Tavares JF, da Silva MS, Diniz Mde F, de Athayde-Filho PF, Barbosa Filho JM. Molecules. 2011; 16:8515–8534.
20. Graf J. Skin Therapy Lett. 2000; 5:3–5.
21. Lee KS, Jeong ES, Heo SH, Seo JH, Jeong DG, Choi YK. Lab Anim Res. 2010; 26:95–102.
22. Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, Leung DY, Holleran W, Uchida Y, Elias PM. J Invest Dermatol. 2008; 128:79–86.
23. Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. Immunol Today. 1998; 19:359–361.
24. Smart JM, Kemp AS. Clin Exp Allergy. 2002; 32:796–802.
25. Tao X, Yin L, Xu L, Peng J. Pharmacol Res. 2018; 137:259–269.
26. Wu S, Xu H, Peng J, Wang C, Jin Y, Liu K, Sun H, Qin J. Biochimie. 2015; 110:62–72.
27. Cao YJ, Xu Y, Liu B, Zheng X, Wu J, Zhang Y, Li XS, Qi Y, Sun YM, Wen WB, Hou L, Wan CP. Am J Chin Med. 2019; 47:423–437.
28. Jegal J, Park NJ, Jo BG, Bong SK, Jegal H, Yang MH, Kim SN. Nutrients. 2018; 10:E1205.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download