Abstract
Piper nigrum L. (Piperaceae), which is a well-known food seasoning, has been used as a traditional medicine for the treatment of vomiting, abdominal pain, diarrhea and anorexia in Korea, China and Japan. Methanol extract from the fruit of P. nigrum was successively partitioned as n-hexane, methylene chloride, ethyl acetate, n-butanol and H2O soluble fractions. Among those fractions the ethyl acetate soluble fraction showed the most potent DPPH radical scavenging activity, and piperine was isolated from the ethyl acetate fraction. To know the antioxidant activity of piperine, we tested the activities of superoxide dismutase (SOD) and catalase together with oxidative stress tolerance and intracellular ROS level in Caenorhabditis elegans. To investigate whether piperine-mediated increased stress tolerance was due to regulation of stress-response gene, we quantified SOD-3 expression using transgenic strain including CF1553. Consequently, piperine enhanced SOD and catalase activities of C. elegans, and reduced intracellular ROS accumulation in a dose–dependent manner. Moreover, piperine-treated CF1553 worms exhibited significantly higher SOD-3::GFP intensity.
Many reports indicate that reactive oxygen species (ROS) play an important role in the defense mechanisms of the body in the biological system. Although low levels of ROS can be beneficial, excessive accumulation can be responsible for the onset of several cellular reactions that can have the harmful effects on the body, causing biomolecule damage. Therefore, it is essential to counter the dangerous effects that ROS can produce by using antioxidants.12 Oxidative stress is a phenomenon caused by an imbalance between production and accumulation of ROS in cells and tissues. Researchers are evaluating medicinal plants to discover and investigate the new antioxidant sources. Actually several antioxidants have been used in recent years for their actual or supposed beneficial effect against oxidative stress, such as vitamin E and polyphenols including flavonoids.3 As a part of investigations to identify the processes of aging and to find cures for aging-associated disorders, several model organisms have been developed, one example of which is the nematode Caenorhabditis elegans is easy to culture with an average lifespan of only about 3 weeks, and its whole genome has been completely sequenced, contributing to the rapid progress in the research of aging.45
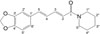
In the course of our continued efforts to find antioxidant compounds related to lifespan-extending, the methanol extract of Piper nigrum L. (Piperaceae) fruit showed antioxidant activity, leading to chemical investigation thereof. Although numerous pharmacological effects of P. nigrum were recognized, including anticancer, analgesic, anti-inflammatory, and antimicrobial activities,678 antioxidant property related to lifespanextending mechanism in C. elegans remains unexplored. Activity-guided purification of the P. nigrum methanol extract enabled us to find the active component, piperine (Fig. 1).To know the antioxidant activity of piperine in C. elegans, we tested the activities of superoxide dismutase (SOD) and catalase together with oxidative stress tolerance, and intracellular ROS level. To investigate whether piperine-mediated increased stress tolerance was due to regulation of stress-response gene, SOD-3 expression was quantified using transgenic strain including CF1553.
Normal-phase TLC was monitored on Merck (Darmstadt, Germany) precoated silica gel F254 plates. TLC spots were visualized under UV and by staining with 10% H2SO4 in ethanol followed by heating. Silica gel column chromatography was carried out using Kiesel gel 60 (230 - 400mesh, Merck, Darmstadt, Germany). Sephadex LH-20 (GE Healthcare, Uppsala, Sweden) was used for performing gel filtration chromatography. HPLC separation employed a JAI-GS310 column (20 × 500mm, JAI, Tokyo, Japan). NMR spectra were obtained from a JEOL JMNEX 600 spectrometer (Tokyo, Japan). The absorbance was measured using a microplate reader (ELISA, Sunrise, Grödig, Austria). Agar, 2′,7′-dichlorodihydrofluoroscein diacetate, juglone, catalase, xanthine, xanthine oxidase, and nitroblue tetrazolium were purchased from Sigma (St. Louis, USA). Peptone and yeast extracts were purchased from BD bioscience (Sparks, USA).
The dried fruit of P. nigrum, purchased from an oriental drug store (Bohwadang, Jeonju, Korea), was identified by one of the authors (Kim, D. K.). A voucher specimen was deposited in the herbarium of the College of Pharmacy, Woosuk University (WSU-17-008). The plant material (500 g) was extracted three times with methanol at 50 ℃. The resulting extract (75 g)was partitioned, providing n-hexane (1.3 g), methylene chloride (12.2 g), ethyl acetate (0.4g), n-butanol (2.3 g), and H2O soluble fractions. DPPH radical scavenging effects of the four fractions was examined. The ethyl acetate fraction showed the most potent DPPH radical scavenging activity (Fig. 2). The ethyl acetate fraction was divided into five subfractions (EA1 – EA5) using Sephadex LH-20 with 100% methanol. Purification trials of the subfraction EA4 afforded piperine (60 mg, yield 0.12%) by successive column chromatographic methods using silica gel (CHCl3:MeOH: H2O, 50 : 10 : 1) and JAI-GS310 (pure MeOH) columns.
1H-NMR (600MHz, CD3OD) δ: 1.60 (4H, m H-2″, 4″), 1.71 (2H, m, H-3″), 3.62 (4H, m, H-1″, 5″), 5.96 (2H, s, O-CH2-O), 6.28 (1H, d, J = 15.0 Hz, H-2), 6.80 (1H, d, J = 8.4 Hz, H-5′), 6.83 (1H, d, J = 16.2 Hz, H-5), 6.90 (1H, dd, J = 11.4, 16.2 Hz, H-4), 6.96 (1H, dd, J = 1.2, 8.4 Hz, H-6′), 7.09 (1H, d, J = 1.2 Hz, H-2′), 7.32 (1H, d, J = 11.4, 15.0 Hz, H-3); 13C-NMR (150 MHz, CD3OD) δ: 167.73 (C-1), 120.65 (C-2), 144.58 (C-3), 126.41 (C-4), 140.15 (C-5), 132.43 (C-1′), 106.68 (C-2′), 149.77 (C-3′), 149.79 (C-4′), 109.36 (C-5′), 23.88 (C-6′), 102.72 (O-CH2-O), 44.54 (C-1″), 26.90 (C-2″), 25.56 (C-3″), 27.89 (C-4″), 48.12 (C-5″). Structure elucidation of piperine was performed by its data compared with the reported data in the literature.9
The worms were grown at 20 ℃ on nematode growth medium (NGM) agar plates in the presence of Escherichia coli strain OP50, as described previously.10 In order to prepare the plates supplemented with the sample to be tested, the solution of piperine in DMSO was inserted into sterilized NGM plates at 50 ℃ to afford a final concentration of 0.1% (v/v). The wild-type Bristol N2 and E. coli OP50 strains were kindly provided by prof. Dong Seok Cha (Woosuk University, Korea).
Oxidative stress tolerance was tested as previously described with minor modification.11 Briefly, on the 7th day of adulthood, the age-synchronized worms were relocated to a 96-well plate containing 1 mM of juglone, as well as various concentrations of piperine. Their survival rates were observed over 35 h.
Homogenates of the worms were prepared for evaluation of enzymatic activities. Briefly, the wild-type N2 worms were harvested on the 5th day of adulthood, and were washed three times with M9 buffer. The worms collected were suspended in buffer (10 mM Tris-HCl, 150 mM NaCl, 0.1 mM EDTA, pH 7.5) and homogenized on ice. The SOD activity was evaluated by spectrophotometric analysis of formazan production derived from the enzymatic reaction between xanthine and xanthine oxidase. The reaction mixture contained 5 µL of the worm homogenates, 120 µL of 0.57mM xanthine, and 0.24mM nitrobluetetrazolium (NBT) in 10 mM phosphate buffer (pH 8.0). After pre-incubation for 5 minutes at room temperature, the reaction was initiated by the addition of 100 µL of xanthine oxidase (0.05 U/ml) followed by incubation for 20 min at 37 ℃. The reaction was stopped when 275 µL of 69 mM SDS was added, and then absorbance of the mixture was measured at 570 nm. The catalase activity was examined using a spectrophotometric method as previously described.12 Briefly, the prepared homogenates were mixed with 25 mM H2O2. After 3 min incubation, absorbance of the mixture was detected at 240 nm. The enzymatic activities were expressed as a percentage of the scavenged amount per control.
Analysis of intracellular ROS generated in the nematodes employed the molecular probe 2′,7′-dichlorodihydrofluoroscein diacetate (H2DCF-DA). An equal number of the wild-type N2 worms was incubated in each NGM agar plate in the presence of piperine. The worms were exposed to 50 µM juglone for 2 h in a 96-well plate on the 4th day of adulthood. Five worms were transferred to each well of a 96-well plate containing 50 µL of M9 buffer. The addition of 50 µL of 25 µM H2DCF-DA solution to the each well resulted in a final concentration of 12.5 µM. Finally, detection of basal fluorescence was carried out immediately in a microplate fluorescence reader at 485 nm (excitation) and 535 nm (emission).
Effect of piperine on the expression of SOD-3 was determined using the age-synchronized transgenic strains CF1553, which contains the green fluorescent protein (GFP)-based reporters SOD-3::GFP. The GFP fluorescence of the mutant was directly observed under a fluorescence microscope (Nikon Eclipse Ni-u, Japan), enabling evaluation of the protein expressions using ImageJ software.1415 All the experiments were carried out in triplicate.
The data obtained from the lifespan and stress resistance assays were plotted using the Kaplan-Meier analysis, statistical significance of which was determined by the log-rank test. The other data are presented as the mean ± standard deviation or standard error of the mean (SEM) as indicated. Statistical significance of the differences between treated and control groups was analyzed by the one-way analysis of variance (ANOVA).
The age-synchronized the wild-type N2 worms were bred on NGM agar plates with or without various concentrations of piperine to examine the effect of piperine under stress condition. The oxidative stress was induced by 1 mM juglone, and under such condition, the duration of survival was prolonged upon treatment with piperine by 20.3 ± 1.4 h (28.3%) and 22.6 ± 1.4 h (42.7%) at 50 µM (p < 0.05) and 100 µM (p < 0.001), respectively, relative to that of the untreated worms (15.8 ± 0.9 h). The maximum survival time for the control group was 27 h, while for the piperine-treated group, the time increased to 31 h and 33 h at 50 µM and 100 µM, respectively (Fig. 3, Table 1).
The superoxide dismutase (SOD) and catalase enzymatic activities were measured spectrophotometrically using prepared worm homogenates. The spectrophotometric measurement was carried out to examine the enzymatic activities of SOD and catalase, using the worm homogenates. The resulting data presented that SOD and catalase activities of the worms were elevated upon treatment with 100 µM piperine by 123.0 ± 3.3 (23.0%, p < 0.001) and 133.3 ± 3.6 (33.3%, p < 0.001), respectively, relative to that of the untreated control worms (Fig. 4).
The ROS levels were measured spectrophotometrically using H2DCF-DA. As shown in Fig. 5, comparison of intracellular ROS levels in the piperine-treated worms with those of untreated controls indicated 88.2 ± 0.6 (11.8%, 100 µM, p < 0.01) decrease in ROS production upon treatment.
In order to investigate whether piperine-mediated increased stress tolerance was due to regulation of stress-response gene, SOD-3 expression was quantified using transgenic strain including CF1553. The fusion of the green fluorescent protein (GFP) to such protein enabled visualization and quantification of the expression level in the mutant. The treatment of CF1553 mutant with piperine increased SOD-3::GFP intensity by 112.9 ± 5.5 (12.9%, p < 0.01) at 100 µM, relative to that of untreated controls (Fig. 6).
Our ongoing investigations to discover lifespan-related antioxidant compounds from natural products led to the isolation of piperine from P. nigrum as an active principle. The C. elegans model is a well-established system for human aging-related research. Treatment of the wild-type strain N2of C. elegans with piperine under a normal culture condition showed the positive activities of several antioxidative experiments. In addition, on the basis of the close relationship reported between oxidative stress resistance and lifespan,16171819 the effect of piperine on stress resistance of the N2 nematode was evaluated under oxidative stress condition. Application of the oxidative stress significantly decreased the average lifespan of the worms, but the addition of piperine displayed elevation of the reduced lifespan in a dose-dependent manner. To elucidate the antioxidant effect of piperine in C. elegans, scavenging activity of antioxidant enzymes that affect free radical/ROS levels was examined in relation to the observed increase in oxidative stress resistance upon treatment. In C. elegans, piperine intake induced dose-dependent increase in the activity levels of the two endogenous antioxidant enzymes SOD and catalase.2021 Also, the intracellular ROS levels of the N2 worms were measured, showing dose-dependent reduction in ROS accumulation by the piperine treatment. In addition, expression level of the stress resistance proteins SOD-3 was determined in transgenic strains using green fluorescent protein (GFP)-based reporter. Higher fluorescence intensity was observed when the transgenic worm was grown in the presence of piperine, indicating that piperine induced SOD-3 expression. To sum up, chemical investigation of P. nigrum led to the isolation of the active metabolite, piperine which showed antioxidant effect in C. elegans. Our further effort to elucidate the mechanism of such action suggested that oxidative stress tolerance of C. elegans was increased upon treatment with piperine via enzyme induction. In addition, expression of the protein SOD-3 was induced by the piperine treatment in the transgenic strains of C. elegans, supporting the proposed mechanism.
In vitro, antioxidant activity of piperine such as quenching or inhibiting free radicals, hydroxyl radicals and ROS was reported.22 In addition, according to the recent study using a cultured human peripheral blood lymphocytes, piperine showed antioxidative effect against cadmium-induced oxidative stress.23 And Umar et al. reported that piperine ameliorates oxidative stress and inflammation in collagen induced arthritis.24 These previous findings are consistent with our results based on the effect on piperine in C. elegns, providing insight into the development of antioxidants related to aging.
Figures and Tables
 | Fig. 2DPPH radical scavenging effects of the MeOH extract and its subsequent fractions from Piper nigrum L. |
 | Fig. 3Oxidative stress tolerance of piperine was determined after the nematodes were transferred to 96-well plates containing 1 mM juglone. Statistical differences between the curves were analyzed by the log-rank test. 4-HBA (4-hydroxybenzoic acid): positive control. |
 | Fig. 4Effects of piperine on the antioxidant enzyme activity of wild type N2 nematodes. (A) SOD activity was shown as a percentage of superoxide scavenged per control. (B) Catalase activity was expressed as a percentage of decrease in residual H2O2 measured by a spectrophotometric method. Differences compared with the control were considered significant at *p < 0.05, **p < 0.01, and ***p < 0.001 by the one-way ANOVA. 4-HBA (4-hydroxybenzoic acid): positive control. |
 | Fig. 5Effects of piperine on the intracellular ROS levels of wild-type N2 nematodes. Intracellular ROS accumulation was examined in a microplate fluorescence reader at 535 nm (emission) and 485 nm (excitation). (A) Plates were read for 120 min. (B) The average percentages of intracellular ROS accumulation were presented. Differences compared with the control were considered significant at *p < 0.05, **p < 0.01, and ***p < 0.001 by the one-way ANOVA. 4-HBA (4-hydroxybenzoic acid): positive control. |
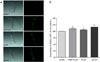 | Fig. 6Effects of piperine on the expression of SOD-3 (CF1553) was determined using transgenic nematodes. (A) Images of SOD-3::GFP expressions of CF1553 nematodes in the presence or absence of piperine. (B) The mean GFP-expressing intensity of CF1553 mutants was expressed as mean ± S.E.M. of values from 90 worms per each experiment. Data are expressed as the mean ± standard deviation of three independent experiments (N=3). Differences compared with the control were considered significant at *p < 0.05 and **p < 0.01 by one-way ANOVA. 4-HBA (4-hydroxybenzoic acid): positive control. |
Table 1
Effects of piperine on the oxidative stress tolerance of C. elegans

Mean lifespan presented as mean ± S.E.M. data. Change in mean lifespan compared with control group (%). Statistical significance of the difference between survival curves was determined by log-rank test using the Kaplan-Meier survival analysis. Differences compared to the control were considered significant at *p < 0.05 and ***p < 0.001. 4-HBA (4-hydroxybenzoic acid): positive control.
References
1. Parveen A, Akash MS, Rehman K, Kyunn WW. Crit Rev Eukaryot Gene Expr. 2016; 26:143–160.
2. Prasad S, Gupta SC, Tyagi AK. Cancer Lett. 2017; 387:95–105.
3. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxid Med Cell Longev. 2017; 2017:8416763.
4. Partridge L, Gems D. Nat Rev Genet. 2002; 3:165–175.
5. Weinert BT, Timiras PS. J Appl Physiol. 2003; 95:1706–1716.
6. Deng Y, Sriwiriyajan S, Tedasen A, Hiransai P, Graidist P. J Ethnopharmacol. 2016; 188:87–95.
7. Tasleem F, Azhar I, Ali SN, Perveen S, Mahmood ZA. Asian Pac J Trop Med. 2014; 7S1:S461–S468.
8. Nikolić MM, Jovanović KK, Marković TL, Marković DL, Gligorijević NN, Radulović SS, Kostić M, Glamoćlija JM, Soković MD. J Pharm Pharmacol. 2017; 69:1606–1614.
10. Brenner S. Genetics. 1974; 77:71–94.
11. Mekheimer RA, Sayed AA, Ahmed EA. J Med Chem. 2012; 55:4169–4177.
12. Aebi H. Methods Enzymol. 1984; 105:121–126.
13. Kim HN, Seo HW, Kim BS, Lim HJ, Lee HN, Park JS, Yoon YJ, Oh JW, Oh MJ, Kwon J, Oh CH, Cha DS, Jeon H. Nat Prod Sci. 2015; 21:128–133.

14. Seo HW, Cheon SM, Lee MH, Kim HJ, Jeon H, Cha DS. Evid Based Complement Alternat Med. 2015; 2015:524878.
15. Waters JC. J Cell Biol. 2009; 185:1135–1148.
16. Kudryavtseva AV, Krasnov GS, Dmitriev AA, Alekseev BY, Kardymon OL, Sadritdinova AF, Fedorova MS, Pokrovsky AV, Melnikova NV, Kaprin AD, Moskalev AA, Snezhkina AV. Oncotarget. 2016; 7:44879–44905.
17. Surco-Laos F, Dueñas M, González-Manzano S, Cabello J, Santos-Buelga C, González-Paramás AM. Food Res Int. 2012; 46:514–521.
18. Liao CY, Kennedy BK. Cell Res. 2016; 26:143–144.
19. Maurya PK, Noto C, Rizzo LB, Rios AC, Nunes SO, Barbosa DS, Sethi S, Zeni M, Mansur RB, Maes M, Brietzke E. Prog Neuropsychopharmacol Biol Psychiatry. 2016; 65:134–144.
20. Heink AE, Parrish AN, Thorgaard GH, Carter PA. Aquat Toxicol. 2013; 144-145:75–82.
21. Homayouni-Tabrizi M, Asoodeh A, Soltani M. J Food Drug Anal. 2017; 25:567–575.
22. Mittal R, Gupta RL. Methods Find Exp Clin Pharmacol. 2000; 22:271–274.
23. Verma N, Bal S, Gupta R, Aggarwal N, Yadav A. J Diet Suppl. 2018; 1–12.
24. Umar S, Golam Sarwar AH, Umar K, Ahmad N, Sajad M, Ahmad S, Katiyar CK, Khan HA. Cell Immunol. 2013; 284:51–59.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download