Abstract
In the present study, we investigated whether quercitrin, quercetin and afzelin derived from Houttuynia cordata affect the production and gene expression of MUC5AC mucin from airway epithelial cells. Confluent NCI-H292 cells were pretreated with quercitrin, quercetin or afzelin for 30 min and then stimulated with epidermal growth factor (EGF) or phorbol 12-myristate 13-acetate (PMA) for 24 h. The MUC5AC mucin gene expression and production were measured by RT-PCR and ELISA, respectively. The results were as follows: (1) Quercitrin, quercetin and afzelin inhibited EGF- and PMA-induced MUC5AC mucin production from NCI-H292 cells; (2) The three natural products also decreased EGF- and PMA-induced MUC5AC mucin gene expression in NCI-H292 cells. These results suggest that quercitrin, quercetin and afzelin showed the regulatory effect on the steps of gene expression and production of mucin, by directly acting on airway epithelial cells.
Mucus present in the human pulmonary system plays an important role in defensive action against invading pathogenic microorganisms, noxious chemicals and particles. This defensive action of pulmonary mucus is attributed to the physicochemical property of mucins i.e. viscoelasticity. Mucins are high molecular weight glycoproteins present in the pulmonary mucus and produced by goblet cells in the surface epithelium as well as mucous cells in the submucosal gland. However, hypersecretion of pulmonary mucus is one of the major symptoms associated with severe respiratory diseases including asthma, chronic bronchitis, cystic fibrosis and bronchiectasis.12 Therefore, it is highly desirable to find the potential activity of regulating the excessive mucin secretion and/or production by the natural products derived from diverse medicinal plants. We have tried to investigate the potential activities of some natural products on mucin secretion and/or production from cultured airway epithelial cells. As a result of our trial, we previously reported that several natural compounds affected mucin secretion and/or production from airway epithelial cells.345 Houttuynia cordata has been utilized empirically for controlling the inflammatory diseases including airway inflammation in folk medicine and diverse biological effects of this medicinal plant have been reported.6 Also, afzelin, quercetin and quercitrin derived from Houttuynia cordata have been reported to exert anti-inflammatory and anti-oxidative activities.7891011 However, to the best of our knowledge, there is no report about the potential effects of quercitrin, quercetin and afzelin derived from Houttuynia cordata on EGF- and PMA-induced gene expression and production of mucin from airway epithelial cells. Among the twenty one or more MUC genes coding human mucins, MUC5AC was reported to be mainly expressed in goblet cells in the airway surface epithelium.212 Therefore, in this study, we checked whether quercitrin, quercetin and afzelin affect MUC5AC mucin production and gene expression from NCI-H292 cells, a human pulmonary mucoepidermoid cell line, which are frequently used for the purpose of studying the airway mucin production and gene expression.131415
All the chemicals and reagents used in this experiment including quercetin (purity: 95.0%), quercitrin (purity: 95.0%) and afzelin (purity: 95.0%) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.) unless otherwise specified.
A preparation of the aqueous and 70% ethanol (EtOH) extracts of Houttuynia cordata and isolation, purification and identification of quercetin, quercitrin and afzelin were performed by analytical chemist, Professor Dr. Jinwoong Kim, in the Laboratory of Pharmacognosy, College of Pharmacy, Seoul National University, Seoul, Korea. For a preparation of the aqueous and 70% EtOH extracts, Houttuynia cordata (80.0 g) were extracted with 500 mL of water and 500 mL of 70% EtOH under reflux (each, for 3 h) and then filtered. The filtrates were evaporated under reduced pressure to give a water extract (18.0 g, 22.5%) and EtOH extract (15.0 g, 18.7%). The isolation and identification of active constituents, quercetin, quercitrin and afzelin, were confirmed by analytical chemist, although we used the same compounds purchased from Sigma-Aldrich for examining the activity on the production and gene expression of MUC5AC mucin, due to the limited amount of the three compounds isolated.
NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, U.S.A.) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) in the presence of penicillin (100 units/mL), streptomycin (100 µg/mL) and HEPES (25mM) at 37 ℃ in a humidified, 5% CO2/95% air, water-jacketed incubator. For serum deprivation, confluent cells were washed twice with PBS and recultured in RPMI 1640 with 0.2% FBS for 24 h.
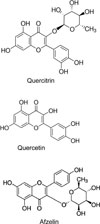
After 24 h of serum deprivation, cells were pretreated with aqueous extract (1 µg/mL, 10 µg/mL and 100 µg/mL), 70% EtOH extract (1 µg/mL, 10 µg/mL and 100 µg/mL), quercitrin, quercetin or afzelin (1, 2, 5 and 10 µM, the chemical structure of each compound can be seen in Fig.1), for 30 min and then treated with epidermal growth factor (EGF) (25 ng/mL) or phorbol 12-myristate 13-acetate (PMA) (10 ng/mL) for 24 h in serum-free RPMI 1640. Quercitrin, quercetin and afzelin were dissolved in dimethylsulfoxide, diluted in PBS and treated in culture medium (final concentrations of dimethylsulfoxide were 0.5%). The final pH values of these solutions were between 7.0 and 7.4. Culture medium and 0.5% dimethylsulfoxide in medium did not affect mucin production and gene expression from NCI-H292 cells. After 24 h, cells were lysed with buffer solution containing 20 mM Tris, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA and protease inhibitor cocktail (Roche Diagnostics, IN, U.S.A.) and collected to measure the production of MUC5AC protein (in 24-well culture plate). The total RNA was extracted for measuring the expression of MUC5AC gene (in 6-well culture plate) by using RT-PCR.
MUC5AC mucin protein was measured by using ELISA. Cell lysates were prepared with PBS at 1:10 dilution, and 100 µL of each sample was incubated at 42 ℃ in a 96-well plate, until dry. Plates were washed three times with PBS and blocked with 2% BSA (fraction V) for 1 h at room temperature. Plates were again washed three times with PBS and then incubated with 100 µL of 45M1, a mouse monoclonal MUC5AC antibody (1:200) (NeoMarkers, CA, U.S.A.), which was diluted with PBS containing 0.05% Tween 20 and dispensed into each well. After 1 h, the wells were washed three times with PBS, and 100 µL of horseradish peroxidase-goat anti-mouse IgG conjugate (1:3,000) was dispensed into each well. After 1 h, plates were washed three times with PBS. Color reaction was developed with 3,3',5,5'-tetramethylbenzidine (TMB) peroxide solution and stopped with 1 N H2SO4. Absorbance was read at 450 nm.
Total RNA was isolated by using Easy-BLUE Extraction Kit (INTRON Biotechnology, Inc. Kyung-gi-do, Korea) and reverse transcribed by using AccuPower RT Premix (BIONEER Corporation, Daejeon, Korea) according to the manufacturer's instructions. 2 µg of total RNA was primed with 1 µg of oligo(dT) in a final volume of 50 µL (RT reaction). 2 µL of RT reaction product was PCR amplified in a 25 µL by using Thermorprime Plus DNA Polymerase (ABgene, Rochester, NY, U.S.A.). Primers for MUC5AC were (forward) 5'-TGA TCA TCC AGC AGG GCT-3' and (reverse) 5'-CCG AGC TCA GAG GAC ATA TGG G-3'. As quantitative controls, primers for Rig/S15 rRNA, which encodes a small ribosomal subunit protein, a housekeeping gene that was constitutively expressed, were used. Primers for Rig/S15 were (forward) 5'-TTC CGC AAG TTC ACC TAC C-3' and (reverse) 5'-CGG GCC GGC CAT GCT TTA CG-3'. The PCR mixture was denatured at 94 ℃ for 2 min followed by 40 cycles at 94 ℃ for 30 s, 60℃ for 30 s and 72 ℃ for 45 s. After PCR, 5 µL of PCR products were subjected to 1% agarose gel electrophoresis and visualized with ethidium bromide under a transilluminator.
As aforementioned in introduction, there is no report about the potential effects of quercitrin, quercetin and afzelin on mucin production and gene expression from airway epithelial cells. Among the twenty one or more MUC genes coding human mucins reported, MUC5AC was mainly expressed in goblet cells in the airway surface epithelium.212 EGF was reported to regulate MUC5AC gene expression in the lung. MUC5AC mRNA expression was increased after binding to the EGF receptor and activation of the mitogen-activated protein kinase (MAPK) cascade.1516 Phorbol 12-myristate 13-acetate (PMA) was reported to stimulate the endogenous activator of protein kinase C (PKC), diacylglycerol (DAG)17 and to be an inflammatory stimulant that can control a gene transcription, 18 cell growth and differentiation.19 PMA also can induce MUC5AC gene expression in NCI-H292 cells. PMA activates a type of isoform of PKC. This activates matrix metalloproteinases (MMPs), which cleave pro-EGFR ligands from the cell surface to become mature EGFR ligands. These ligands bind to the EGF receptor, provoking the phosphorylation of its intracellular tyrosine kinase. This leads to activation of MEK leading to ERK activation. Following is the activation of the transcription factor, Sp1, and binding of the factor to specific sites with the MUC5AC gene promoter. Eventually, the promoter is activated and produced the gene transcription and translation to MUC5AC mucin protein.18 Based on these reports, we investigated the effects of quercitrin, quercetin and afzelin on EGF- or PMA-induced MUC5AC mucin production and gene expression from NCI-H292 cells, a human pulmonary mucoepidermoid cell line. First of all, we tried to investigate the potential effect of total extract of Houttuynia cordata on PMA-induced MUC5AC mucin production. As shown in Fig. 2, aqueous extract of Houttuynia cordata inhibited PMA-induced MUC5AC mucin production from NCI-H292 cells, at the highest concentration. The amounts of mucin in the cells of aqueous extract of Houttuynia cordata-treated cultures were 100 ± 9%, 331 ± 17%, 334 ± 17%, 291 ± 29% and 231 ± 13% for control, 10 ng/mL of PMA alone, PMA plus aqueous extract of Houttuynia cordata 1 µg/mL, PMA plus aqueous extract of Houttuynia cordata 10 µg/ mL and PMA plus aqueous extract of Houttuynia cordata 100 µg/mL, respectively (A). Also, EtOH extract of Houttuynia cordata inhibited PMA-induced MUC5AC mucin production from NCI-H292 cells. The amounts of mucin in the cells of EtOH extract of Houttuynia cordata treated cultures were 100 ± 9%, 331 ± 17%, 316 ± 10%, 315 ± 31% and 81 ± 2% for control, 10 ng/mL of PMA alone, PMA plus EtOH extract of Houttuynia cordata 1 µg/mL, PMA plus EtOH extract of Houttuynia cordata 10 µg/mL and PMA plus EtOH extract of Houttuynia cordata 100 µg/mL, respectively (B). This result means that total extract of Houttuynia cordata showed the possibility of regulating airway mucin overproduction, observed in various pulmonary inflammatory diseases. Thus, we decided to examine a possible regulatory effect of the major constituents derived from Houttuynia cordata. As can be seen in Fig. 3 & Fig. 5, quercitrin, quercetin and afzelin inhibited PMA- and EGF-induced MUC5AC mucin production from NCI-H292 cells. The amounts of mucin in the cells of quercitrin-treated cultures were 100 ± 4%, 239 ± 13%, 176 ± 11%, 133 ± 11%, 97 ± 2% and 60 ± 8% for control, 10 ng/mL of PMA alone, PMA plus quercitrin 1 µM, PMA plus quercitrin 2 µM, PMA plus quercitrin 5 µM and PMA plus quercitrin 10 µM (Fig. 3 (A)). The amounts of mucin in the cells of quercetin-treated cultures were 100 ± 7%, 328 ± 13%, 296 ± 11%, 246 ± 8%, 151 ± 9% and 115 ± 2% for control, 10 ng/mL of PMA alone, PMA plus quercetin 1 µM, PMA plus quercetin 2 µM, PMA plus quercetin 5 µM and PMA plus quercetin 10 µM (Fig. 3 (B)). The amounts of mucin in the cells of afzelin-treated cultures were 100 ± 9%, 266 ± 3%, 243 ± 23%, 177 ± 15%, 150 ± 4% and 64 ± 4% for control, 10 ng/mL of PMA alone, PMA plus afzelin 1 µM, PMA plus afzelin 2 µM, PMA plus afzelin 5 µM and PMA plus afzelin 10 µM (Fig. 3 (C)). Also, the amounts of mucin in the cells of quercitrin-treated cultures were 100 ± 3%, 234 ± 12%, 232 ± 4%, 205 ± 14%, 196 ± 1% and 110 ± 1% for control, 25 ng/mL of EGF alone, EGF plus quercitrin 1 µM, EGF plus quercitrin 2 µM, EGF plus quercitrin 5 µM and EGF plus quercitrin 10 µM (Fig. 5 (A)). The amounts of mucin in the cells of quercetintreated cultures were 100 ± 6%, 353 ± 18%, 258 ± 7%, 193 ± 5%, 162 ± 9% and 121 ± 4% for control, 25 ng/mL of EGF alone, EGF plus quercetin 1 µM, EGF plus quercetin 2 µM, EGF plus quercetin 5 µM and EGF plus quercetin 10 µM (Fig. 5 (B)). The amounts of mucin in the cells of afzelin-treated cultures were 100 ± 1%, 228 ± 4%, 239 ± 7%, 236 ± 2%, 193 ± 8% and 113 ± 3% for control, 25 ng/mL of EGF alone, EGF plus afzelin 1 µM, EGF plus afzelin 2 µM, EGF plus afzelin 5 µM and EGF plus afzelin 10 µM (Fig. 5 (C)). At the same time, the gene expression of MUC5AC mucin induced by PMA or EGF in NCI-H292 cells was inhibited by pretreatment with quercitrin, quercetin or afzelin, respectively (Fig. 4 and Fig. 6)
These results suggest that quercitrin, quercetin and afzelin showed the regulatory effect on the steps of gene expression and production of mucin, by directly acting on airway epithelial cells. The underlying mechanisms of action of quercitrin, quercetin and afzelin on MUC5AC mucin production and gene expression are not clear at present, although we are investigating whether the three natural products act as potential regulators of the MAPK cascade after ligand binding to the EGF receptor and/or potential regulators of NF-kB signaling pathway, in mucin-producing NCI-H292 cells.
Taken together, the inhibitory action of quercitrin, quercetin and afzelin on airway mucin production and gene expression might explain, at least in part, the traditional use of Houttuynia cordata as an anti-inflammatory agent for pulmonary inflammatory diseases, in traditional oriental medicine. We suggest it is valuable to find the natural products that have specific inhibitory effects on mucin production and gene expression - in view of both basic and clinical sciences - and the result from this study suggests a possibility of developing quercitrin, quercetin or afzelin as a candidate for the new efficacious mucoregulators for pulmonary diseases, although further studies are essential.
Figures and Tables
Fig. 2
Effect of aqueous or ethanolic extract of Houttuynia cordata on PMA-induced MUC5AC mucin production from NCI-H292 cells.
NCI-H292 cells were pretreated with varying concentrations of aqueous (A) or ethanolic (B) extract of Houttuynia cordata for 30 min and then stimulated with PMA (10 ng/mL) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production by ELISA. Each bar represents a mean ± S.E.M. of 3 culture wells in comparison with that of control set at 100%. Three independent experiments were performed and the representative data were shown.
* significantly different from control (p<0.05).
+ significantly different from PMA alone (p<0.05).
(cont: control, HC: Houttuynia cordata, concentration unit is µg/ mL.)

Fig. 3
Effect of quercitrin, quercetin or afzelin on PMA-induced MUC5AC mucin production from NCI-H292 cells.
NCI-H292 cells were pretreated with varying concentrations of quercitrin (A), quercetin (B) or afzelin (C) for 30 min and then stimulated with PMA (10 ng/mL) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production by ELISA. Three independent experiments were performed and the representative data were shown. Each bar represents a mean ± S.E.M. of three culture wells in comparison with that of control set at 100%.
* significantly different from control (p<0.05).
+ significantly different from PMA alone (p<0.05).
(cont: control, Q: quercetin, Qc: quercitrin, A: afzelin, concentration unit is µM.)
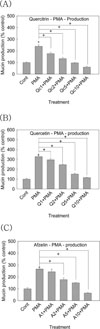
Fig. 4
Effect of quercitrin, quercetin or afzelin on PMA-induced MUC5AC mRNA expression in NCI-H292 cells.
NCI-H292 cells were pretreated with varying concentrations of quercitrin, quercetin or afzelin for 30 min and then stimulated with PMA (10 ng/mL) for 24 h. MUC5AC mRNA expression was measured by RT-PCR. Three independent experiments were performed and the representative data were shown.
(cont: control, Q: quercetin, Qc: quercitrin, A: afzelin, concentration unit is µM.)
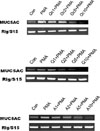
Fig. 5
Effect of quercitrin, quercetin or afzelin on EGF-induced MUC5AC mucin production from NCI-H292 cells.
NCI-H292 cells were pretreated with varying concentrations of quercitrin (A), quercetin (B) or afzelin (C) for 30 min and then stimulated with EGF (25 ng/mL) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production by ELISA. Three independent experiments were performed and the representative data were shown. Each bar represents a mean ± S.E.M. of three culture wells in comparison with that of control set at 100%.
* significantly different from control (p<0.05).
+ significantly different from EGF alone (p<0.05).
(cont: control, Q: quercetin, Qc: quercitrin, A: afzelin, concentration unit is µM.)

Fig. 6
Effect of quercitrin, quercetin or afzelin on EGF-induced MUC5AC mRNA expression in NCI-H292 cells.
NCI-H292 cells were pretreated with varying concentrations of quercitrin, quercetin or afzelin for 30 min and then stimulated with EGF (25 ng/mL) for 24 h. MUC5AC mRNA expression was measured by RT-PCR. Three independent experiments were performed and the representative data were shown.
(cont: control, Q: quercetin, Qc: quercitrin, A: afzelin, concentration unit is µM.)

References
1. Lee CJ, Park SH, Ko KH, Kim KC. Inflamm Res. 2002; 51:490–494.
2. Voynow JA, Rubin BK. Chest. 2009; 135:505–512.
3. Heo HJ, Kim C, Lee HJ, Kim YS, Kang SS, Seo UK, Kim YH, Park YC, Seok JH, Lee CJ. Phytother Res. 2007; 21:462–465.
4. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Phytother Res. 2009; 23:1458–1461.
5. Kim KD, Lee HJ, Lim SP, Sikder A, Lee SY, Lee CJ. Phytother Res. 2012; 26:1301–1307.
6. Shingnaisui K, Dev T, Manna P, Kalita J. J. Ethnopharmacol. 2018; 220:35–43.
7. Chang JH, Song KJ, Kim HJ, Kim JH, Kim NH, Kim KS. Am J Rhinol Allergy. 2010; 24:e59–e62.
8. Kwon SH, Nam JI, Kim SH, Kim JH, Yoon JH, Kim KS. Phytother Res. 2009; 23:1708–1712.
9. Dönder Y, Arikannn TB, Baykan M, Akyüz M, Öz AB. Asian J Surg. 2018; 41:543–550.
10. Choe KI, Kwon JH, Park KH, Oh MH, Kim MH, Kim HH, Cho SH, Chung EK, Ha SY, Lee MW. Molecules. 2012; 17:11484–11494.
11. Zhou W, Nie X. Mol Med Rep. 2015; 12:71–76.
12. Rogers DF, Barnes PJ. Ann Med. 2006; 38:116–125.
13. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, Nadel JA, Prince A, Basbaum CB. Proc Natl Acad Sci USA. 1997; 94:967–972.
14. Shao MX, Ueki IF, Nadel JA. Proc Natl Acad Sci USA. 2003; 100:11618–11623.
15. Takeyama K, Dabbagh K, Lee HM, Agustí C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Proc Natl Acad Sci USA. 1999; 96:3081–3086.
16. Takeyama K, Dabbagh K, Shim JJ, Dao-Pick T, Ueki IF, Nadel JA. J Immunol. 2000; 164:1546–1552.
17. Hong DH, Petrovics G, Anderson WB, Forstner J, Forstner G. Am J Physiol. 1999; 277:G1041–G1047.
18. Hewson CA, Edbrooke MR, Johnston SL. J Mol Biol. 2004; 344:683–695.
19. Park SJ, Kang SY, Kim NS, Kim HM. Immunopharmacol Immunotoxicol. 2002; 24:211–226.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download