Abstract
A new isoprenylated stilbene, flavestinK (1) together with two known isoprenylated stilbenes, flavestin B (2), flavestin G (3), and two isoprenilated flavanones, 4-O-methyl-8-isoprenylnaringenin (4) and 8-isoprenyl-5,7-dihydroxyflavanone (5) were isolated from the leaves of Macaranga recurvata Gage. All of the structures have been determined based on HRESIMS, 1D and 2D NMR spectral data. All of the isolated compounds were evaluated for their cytotoxicity against three human cancer cells (HeLa, T47D and WiDr). Compound 1 showed higher activity than doxorubicin against HeLa cells with IC50 value of 13.1 µg/mL.
Macaranga recurvata Gage (Euphorbiaceae), locally known as ‘Mahang merah’ is one pioneer plant and found endemic in Kalimantan Island, Indonesia. The genus Macaranga have been showed a number of phenolic compounds, predominantly flavonoids and stilbenes with terpenylated side chain (isoprenyl, geranyl and farnesyl) in aromatic ring.1234 Based on previously report, two isoprenylated dihydroflavonols, macarecurvatins A and B from the leaves of M. recurvata showed cytotoxicities against murine leukemia.5 Isoprenylation of flavonoids and stilbenes seems to be a key factor to enhance their cytotoxicity.
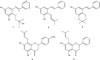
In this research paper, we desiderate to report the isolation of a new isoprenylated stilbene, flavestin K (1) along with four known compounds, flavestin B (2), flavestin G (3), 4′-O-methyl-8-isoprenylnaringenin (4) and 8-isoprenyl-5,7-dihydroxyflavanone (5) from the leaves of M. recurvata (Fig. 1). The cytotoxic activities of compounds 1-5 against three human cancer cells (HeLa, T47D and WiDr) are also reported.
1D NMR (1H and 13C), 2D NMR (HMQC and HMBC) spectra were recorded with a JEOL JNM-ECA 400 FT NMR spectrometer operating at 400 MHz usingdeuterated solvent (peaks: δH 2.04 and δC 29.8 for acetone-d6 as reference standard). High resolution mass spectra were measured on an ESI-TOF Waters LCT Premier X Emass spectrometer. All of compounds were dissolved in methanol and were measured by UV spectrophotometer Shimadzu 1900. The functional group of compounds in KBr were measured by IR Tracer-100 Shimadzu FT IR spectrophotometer. Column chromatography (CC) was performed using Si gel 60 G and centrifugal planar chromatography (CPC) was performed using Si gel 60 PF254. TLC analysis was performed using on pre-coated Si gel 60 GF 254 0.25 mm thickness plates.
The leaves of M. recurvata were collected from Muara Teweh, North Barito Districts, East Kalimantan, Indonesia on Feb. 2018, and identified by senior botanist Mr. Ismail Rachman from the Herbarium Bogoriense, Center of Biological Research and Development, National Institute of Science, Bogor, Indonesia.
The air-dried and powdered leaves of M. recurvata (3.5 kg) were extracted with MeOH (2 × 15 L) at room temperature for two days, and after evaporation gave the viscous concentrated of MeOH extract (210 g). The suspended solids was redissolved in MeOH-H2O (9:1) and then partitioned with C6H14 (45 g) and EtOAc (22 g), successively.
The EtOAc extract (20 g) was further fractionated by column chromatography on silica gel (800 g) eluted with C6H14-EtOAc (from 9:1 to 1:1) by gradient elutionto give five major fractions A-E. Fraction B (2.10 g) was separated by sephadex LH-20 eluted with MeOH gave subfractions B1-B2. Subfraction B2 was purified by centrifugal planar chromatography using C6H14-CHCl3 (from 7:3 to 3:7) to yield compounds 4 (30 mg), and 5 (19 mg). Fraction C (2.8 g) was subjected to sephadex LH-20 and eluted with MeOH gave three subfractions C1-C3. Subfraction C1 was purified by CPC using C6H14-EtOAc (from 9:1 to 4:1) gave compound 2 (40 mg) and purification of subfraction C2 by the same methods using n-hexane-diisopropylether (from 1:1 to 1:4) to yield compounds 1 (5mg), and 3 (21mg).
Yellow solid, UV (MeOH) λmax nm (log ε): 212 (4.49), and 293 (4.26). IR (KBr) νcm−1: 3400, 1604, 1521, 1438 and 1033. 1H and 13C NMR see Table 1. HRESIMS: m/z [M-H]− calcd. for C19H21O3 297.1491, found 297.1489.
Amorphous powder, UV (MeOH) λmax nm (log ε) : 215 (4.39), and 303 (4.10). IR (KBr) νcm−1: 3368, 1608, 1530, 1463 and 1028. HRESIMS: m/z [M-H]− calcd. for C19H19O2 279.1523, found 279.1519. The 1H and 13C NMR spectral data were compared and consistent with the published data.6
Amorphous powder, UV (MeOH) λmax nm (log ε): 214 (4.42), and 295 (4.17). IR (KBr) νcm−1: 3340, 1602, 1548, 1459 and 1033. HRESIMS: m/z [M-H]− calcd. for C19H19O2 279.1489, found 279.1480. The 1H and 13C NMR spectral data were compared and consistent with the published data.6
White solid, m.p. 169 – 170℃. UV (MeOH) λmax nm (log ε): 214 (4.46), and 293 (4.67). IR (KBr) νcm−1: 3425, 1640, 1515, 1448 and 1170. HRESIMS: m/z [M-H]− calcd. for C21H21O5 353.1457, found 353,1452. The 1H and 13C NMR spectral data were compared and consistent with the published data.7
White solid, m.p. 165 – 167℃. UV (MeOH) λmax nm (log ε) : 224 (4.49), and 294 (4.73). IR (KBr) νcm−1: 3418, 1638, 1520, 1446 and 1168. HRESIMS: m/z [M-H]− calcd. for C20H19O4 323.1362, found 323.1365. The 1H and 13C NMR spectral data were compared and consistent with published data.8
Compounds 1 – 5 were appraised for their cytotoxicity toward HeLa (human cervical carcinoma), T47D (human breast cancer), and WiDr (human colon carcinoma) according to the MTT method as well as doxorubicin as the positive control.9101112 Briefly, before the compounds were added, cells were cultured in 96-well at a density of 3 × 104 cells/well and incubated at 37℃ for 24 h. Compounds 1 – 5 with variations in concentration (100, 30, 10, 3, 1, 0.3, and 0.1 µg/mL) with triplicate were added to each well and incubated at 37℃ for 48 h. After incubation, it was added MTT and let for 4 h, and the inhibition of cells by each of compounds 1 – 5 were recorded with microplate reader spectrophotometer at λ 570 nm. The IC50 values of all compounds were calculated by regression analysis.
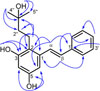
Flavestin K (1) obtained as yellow solid, and the chemical formula C19H22O3 was deduced by HRESIMS spectra with ion peak [M-H]− at m/z 297.1489 (calcd. for 297.1491). The UV spectrum showed two maximum absorption at λmax (log ε) 212 (4.49), and 293 (4.26) nm characteristic for a stilbene chromophore.13 The IR spectrum displayed absorption band for hydroxyl (3400 cm−1), and aromatic ring (1521 – 1438 cm−1), respectively. The 1H NMR spectrum of compound 1 (Table 1) exhibited two doublets (J = 16.1 Hz) at δH 7.52 (H-α) and δH 6.98 (H-β) provided the evidence for a trans 1,2-disubstituted ethene connecting with two aromatic rings, revealed that compound 1 was (E)-stilbene.14 The existence of meta-coupling doublets (J = 2.4 Hz) at δH 6.35 (H-4) and δH 6.69 (H-6) is characteristic for a 1,2,3,5-tetrasubstituted benzene. The presence of three multiplet signals at δH 7.57 (H-2′/H-6′), δH 7.34 (H-3′/H-5′) and δH 7.24 (H-4′) is consistent for a monosubstituted benzene. 1H NMR spectrum of 1 also disclosed information two phenolic hydroxyl groups at δH 8.19 (3-OH), and δH 7.99 (5-OH). The proton signals for a 2-methylbutan-2-ol unit were observed at δH 3.58 (3″-OH), 2.83 (H2-1″), 1.66 (H2-2″) and 1.25 (H3-4″ and H3-5″) in the 1H NMR spectrum. Sixteen carbon signals were observed in 13C NMR spectrum. The carbon NMR signals including two oxyaryl carbon signals (δC 156.9 and δC 156.7) recommended that compound 1 has a structure of pinosylvin (E-3,5-dihydroxystilbene) bearing a 2-methylbutan-2-ol group.15 Location of a 2-methylbutyl-2-ol group in pinosylvin structure was established by 2D NMR spectra (HMQC and HMBC) (Fig. 2). The H-α proton (δH 7.52) of the ethene group correlated with a methine carbon at δC 104.4 (C-6) and two quaternary carbons at δC 120.2 (C-2) and at δC 138.2 (C-1′) in the HMBC spectrum of 1. The HMBC correlations of the methylene proton signal at δH 2.83 (H-1″) to δC 138.8 (C-1), 120.2 (C-2), 156.9 (C-3), 45.0 (C-2″), and 70.5 (C-3″) indicated that a 2-methylbutyl-2-ol chain was located at C-2. The presence of 2-methylbutan-2-ol chain was further supported by HMBC correlations between the hydroxyl proton signal at δH 3.58 (3″-OH) and the carbon signals at δC 29.9 (C-4″ and C-5″), 45.0 (C-2″) and 70.5 (C-3″). The structure of compound 1 was thus identified as 2-(3-hydroxy-3-methylbutyl)-pinosylvin. We designated the compound 1 as flavestin K.
The cytotoxicity activityof compounds 1 – 5 were appraised towards HeLa (human cervical carcinoma), T47D (human breast cancer), and WiDr (human colon carcinoma) by MTT method as well as doxorubicin (positive control).16 Isoprenylated stilbenes (1, 2 and 3) more active than isoprenylated flavonoids (4 and 5) (Table 2). Compound 1 exhibited higher activity than doxorubicin against HeLa with IC50 value of 13.1 µg/mL. However, compounds 4 – 5 were inactive against HeLa, T47D, and WiDr cells.
Figures and Tables
Acknowledgments
This study was supported by Penelitian Hibah Mandat, 2019, Universitas Airlangga, Surabaya, Indonesia.
References
1. Syah YM, Ghisalberti EL. Nat Prod Commun. 2010; 5:219–222.
2. Kamarozaman AS, Ahmat N, Isa SNM, Hafiz ZZ, Adenan MI, Yusof MIM, Azmin NFN, Latip J. Phytochem Lett. 2019; 30:174–180.
3. Yang DS, Wei JG, Peng WB, Wang SM, Sun C, Yang YP, Liu KC, Li XL. Fitoterapia. 2014; 99:261–266.
4. Tanjung M, Hakim EH, Syah YM. Chem Nat Compd. 2017; 53:215–218.
5. Tanjung M, Hakim EH, Elfahmi , Latip J, Syah YM. Nat Prod Commun. 2012; 7:1309–1310.
6. Kusano G, Koguchi S, Shibano M, Takahashi K, Okada Y, Coskun M, Ozgen U, Erdurak CS, Okuyama T. Nat Med. 2002; 56:129–135.
7. Marliana E, Tjahjandarie TS, Tanjung M. Der Pharm Lett. 2015; 7:153–156.
8. Dong X, Liu Y, Yan J, Jiang C, Chen J, Liu T, Hu Y. Bioorg Med Chem. 2008; 16:8151–8160.
9. Saputri RD, Tjahjandarie TS, Tanjung M. Nat Prod Sci. 2018; 24:155–158.
10. Tanjung M, Rachmadiarti F, Saputri RD, Tjahjandarie TS. Nat Prod Res. 2018; 32:1062–1067.
11. Mah SH, Ee GCL, Teh SS, Sukari MA. Nat Prod Res. 2015; 29:98–101.
12. Sigmond J, Backus HHJ, Wouters D, Temmink OH, Jansen G, Peters GJ. Biochem Pharmacol. 2003; 66:431–438.
13. Lee SK, Lee HJ, Min HY, Park EJ, Le KM, Ahn YH, Cho YJ, Pyee JH. Fitoterapia. 2005; 76:258–260.
14. Tanjung M, Juliawaty LD;, Syah YM. Fitoterapia. 2018; 126:74–77.
15. Pailee P, Sangpetsiripan S, Mahidol C, Ruchirawat S, Prachyawarakorn V. Tetrahedron. 2015; 71:5562–5571.
16. Marliana E, Astuti W, Kosala K, Hairani R, Tjahjandarie TS, Tanjung M. Asian J Chem. 2018; 30:795–798.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download