Abstract
Three Diels-Alder type adducts, guangsangon E (1), chalcomoracin (2) and sorocein I (3) were isolated from hairy root cultures of Morus macroura. The structures of the isolated compounds (1 – 3) were determined by spectroscopic method (NMR and MS), and spectral comparison to literature. Cytotoxic activities of the isolated compounds (1 – 3) were investigated against P-388 murine leukemia cell line. Guangsangon E (1) showed the most potent cytotoxicity against P-388 murine leukemia cell line with IC50 value of 2.75 ± 0.32 µg/mL. To the best of our knowledge, guangsangon E (1) and sorocein I (3) were reported for the first time from the tissue cultures of M. macroura.
Morus macroura, locally known as ‘Andalas’, is a rare plant found and native to the Malesian region.1 It is a tree growing up to 35m and found at altitudes of 900 – 1600m.1 In the region, the plant has been valued for the high quality of the wood due to its resistance to insects. Unfortunately, destruction of Indonesian forest threatens the existence and future study of this plant, especially the investigation of secondary metabolites. Plant tissue culture is a method that can be applied to solve the obstacle in investigating compounds in this plant.
Previous phytochemical studies on M. macroura revealed the isolation of Diels-Alder type adducts.234567 Two new benzofuran derivatives, macrourins A and B, were also isolated from this plant.8 Our previous works on the chemical constituents of this plant have resulted in the isolation of other phenolic compounds, including andalasin A, andalasin B, quercimeritrin, moracin B, moracin P, oxyresveratrol, and mulberroside C.910 Furthermore, our work on hairy root cultures of M. macroura afforded chalcomoracin and mulbereofuran P, Diels-Alder type adducts.11
Hairy root culture is one of the interesting methods in the plant tissue cultures since the culture able to grow fast and produce secondary metabolites as well. In our previous work, hairy root cultures of M. macroura were successfully produced via transformation of the plant leaves using Agrobacterium rhizogenes.11 Furthermore, the effect of Saccharomyces cerevisiae extract on production of secondary metabolites of M. macroura hairy root cultures had been also investigated.11 In continuation of our studies on these elicited hairy root cultures, here we report the isolation and identification of Diels-Alder type adducts from the methanol extract of the cultures. The isolated compounds (1 – 3) were tested for cytotoxicity against P-388 murine leukemia cell line.
IR spectra were recorded on a Perkin Elmer spectrum One FT-IR spectrometer. UV spectra were recorded on a Varian Corry 100 Conc spectrometer. NMR spectra were recorded on JEOL JNM A 5000 at 400 MHz (1H) and 100 MHz (13C). MS spectrum of compound 1 was recorded on an ESI-TOF Waters Premier LCT XE mass spectrometer. Meanwhile, MS spectra of compound 2 and compound 3 were recorded using an API 3000 mass spectrometer (Applied Biosystems/MDS Sciex). Vacuum liquid chromatography (VLC) and radial chromatography were carried out using Merck Silica gel 60 GF254. Column chromatography was performed using Sephadex LH-20. Flash column chromatography was carried out by Merck Silica gel 60 (70–230 mesh). TLC was performed using Merck precoated silica gel F254 plates.
Hairy root transformation of M. macroura by A. rhizogenes had been assisted by Research Center of Biotechnology, Indonesian Institute of Sciences (LIPI). Hairy root cultures of M. macroura were grown in 50 mL of Murashige Skoog (MS) media with 0.5 ppm of indol-3-buteric acid (IBA) at room temperature and shaken at 100 rpm. Subculture (propagation) of the hairy root cultures was carried out at week 4 until the number of the cultures are enough for the elicitation. Fig. 1 showed the growth of hairy root cultures of M. macroura from week 0 till week 4. Elicitation was conducted by adding 500 µL of S. cerevisiae (2.5% w/v) into the hairy root cultures at the week 8 and followed by shaking at 100 rpm for 7 days and then harvested.
S. cerevisiae was obtained from Microbiology Laboratory, School of Life Sciences and Technology, Bandung Institute of Technology, Indonesia. S. cerevisiae was grown in 50 mL of glucose-yeast extract media (GYE) at room temperature and shaken at 100 rpm. The cultures of S. cerevisiae were subcultivated every 2 days by transferring 10 mL of the culture into 40 mL of the fresh liquid medium. The cultures were harvested at 16 hours old. The supernatant was removed, and the cells were dried and grinded into powders.
Dried powdered of hairy roots of M. macroura (35.5 g) were macerated with MeOH yielding 7.9 g of dried extract. The MeOH extract was fractionated with vacuum liquid chromatography (hexane-EtOAc, gradient concentrations) into seven fractions (A-H). Fraction C was subjected to silica gel radial chromatography (EtOAc-chloroform, 2:8) yielding 7 fractions. Fraction C7 was successively purified by Sephadex LH-20 column chromatography and flash silica gel column chromatography (hexane-acetone, 6:4), afforded compound 1 (8 mg) and compound 2 (20 mg). Fraction E was successively purified by silica gel radial chromatography (chloroform-methanol, 9:1) and flash silica gel column chromatography (hexane-acetone, 6:4) yielding compound 3 (5 mg).
Guangsangon E (1): Brown amorphous gum; UV (MeOH) λmax (log ε) = 203 (8.45), 225 (7.34), 282 (5.61), 323 (4.93) nm; IR νmax (KBr) = 3445, 2927, 1619, 1497, 1461 cm−1; 1H NMR (500 MHz, acetone-d6): δ (ppm) = 1.56 (3H, s, H-24″), 1.67 (3H, s, H-25″), 1.89 (3H, s, H-7″), 2.07 (1H, m, H-6a″), 2.46 (1H, m, H-6b″), 3.19 (2H, d, J = 7.0 Hz, H-21″), 3.67 (1H, m, H-5″), 4.38 (1H, br s, H-3″), 4.68 (1H, dd, J = 6.5, 7.0 Hz, H-4″), 5.12 (1H, t, J = 7.0 Hz, H-22″), 5.55 (1H, s, H-2″), 6.14 (1H, dd, J = 1.5, 8.5 Hz, H-19″), 6.26 (1H, d, J = 1.5 Hz, H-17″), 6.33 (1H, d, J = 2.0 Hz, H-4′), 6.42 (1H, d, J = 8.5 Hz, H-13″), 6.81 (1H, s, H-7), 6.83 (1H, d, J = 2.0 Hz, H-2′), 6.83 (1H, d, J = 2.0 Hz, H-6′), 6.88 (1H, d, J = 8.5 Hz, H-20″), 7.04 (1H, s, H-3), 7.45 (1H, s, H-4), 8.03 (1H, d, J = 8.5 Hz, H-14″), 13.03 (1H, s, HO-10″); 13C NMR (125 MHz, acetone-d6): δ (ppm) = 17.9 (CH3, C-25″), 22.3 (CH2, C-21″), 23.8 (CH3, C-7″), 25.9 (CH3, C-24″), 33.5 (CH, C-5″), 37.4 (CH2, C-6″), 37.9 (CH, C-3″), 48.5 (CH, C-4″), 97.2 (CH, C-7), 102.6 (CH, C-3), 103.4 (CH, C-17″), 103.8 (CH, C-4′), 103.8 (CH, C-2′), 103.9 (CH, C-6′), 107.3 (CH, C-13″), 107.6 (CH, C-19″), 115.4 (C-11″), 115.8 (C-9″), 121.9 (C-3α), 123.2 (C-15″), 123.6 (CH, C-4), 123.8 (CH, C-22″), 124.5 (CH, C-2″), 125.6 (C-5), 127.1 (C-8″), 129.8 (CH, C-20″), 129.8 (C-1′), 130.6 (CH, C-14″), 131.2 (C-23″), 135.2 (C-1″), 155.1 (C-7α), 155.1 (C-16″), 155.3 (C-2), 156.4 (C-6), 157.3 (C-18″), 159.8 (C-3′), 159.8 (C-5′), 161.9 (C-12″), 163.9 (C-10″). HRESI-MS: m/z 649.2426 (calcd. 649.2438 for C39H37O9). The data were accordance with the literature.3
Chalcomoracin (2): Yelow amorphous gum; UV (MeOH) λmax (log ε) = 203 (7.45), 319 (7.49), 333 (3.84) nm; IR νmax (KBr) = 3349, 2913, 1669, 1619, 1489, 1440 cm−1; 1H NMR (400 MHz, acetone-d6): δ (ppm) = 1.56 (3H, s, H-25″), 1.70 (3H, s, H-24″), 1.95 (3H, s, H-7″), 2.21 (1H, m, H-6a″), 2.52 (1H, m, H-6b″), 3.25 (2H, d, J = 6.9 Hz, H-21″), 3.75 (1H, t, J = 4.5 Hz, H-5″), 4.11 (1H, br s, H-3″), 4.63(1H, t, J = 4.5 Hz, H-4″), 5.14 (1H, t, J = 6.9 Hz, H-22″), 5.77 (1H, br s, H-2″), 6.30 (1H, dd, J = 8.0, 2.1 Hz, H-19″), 6.43 (1H, d, J = 9.3 Hz, H-13″), 6.50 (1H, d, J = 2.1 Hz, H-17″), 6.76 (1H, br s, H-2′), 6.76 (1H, br s, H-6′), 6.76 (1H, m, H-5), 6.92 (1H, br s, H-3), 6.92 (1H, br s, H-7), 6.98 (1H, d, J = 8.0 Hz, H-20″), 7.34 (1H, d, J = 8.1 Hz, H-4), 8.44 (1H, d, J = 9.3 Hz, H-14″); 13C NMR (100 MHz, acetone-d6): δ (ppm) = 17.8 (CH3, C-25″), 22.2 (CH2, C-21″), 23.8 (CH3, C-7″), 25.8 (CH3, C-24″), 32.2 (CH2, C-6″), 33.1 (CH, C-3″), 36.5 (CH, C-5″), 47.8 (CH, C-4″), 98.3 (CH, C-7), 101.8 (CH, C-3), 103.4 (CH, C-17″), 104.8 (CH, C-2′), 104.8 (CH, C-6′), 107.4 (CH, C-19″), 108.1 (CH, C-13″), 113.0 (CH, C-5), 113.4 (C-9″), 115.8 (C-4′), 116.6 (C-11″), 121.8 (CH, C-4), 121.8 (C-15″), 122.6 (C-3a), 123.1 (CH, C-22″), 124.4 (CH, C-2″), 128.7 (CH, C-20″), 130.9 (C-1′), 131.5 (C-23″), 132.1 (CH, C-14″), 133.8 (C-1″), 155.4 (C-7a), 156.3 (C-2), 156.5 (C-3′), 156.5 (C-5′), 156.6 (C-16″), 157.8 (C-6), 157.8 (C-18″), 163.3 (C-12″), 164.4 (C-10″), 209.7 (C-8″); ESI-MS: m/z 648.5 (C39H36O9). The data were in agreement with the literature.1213
Sorocein I (3): Purple amorphous gum; UV (MeOH) λmax (log ε) = 205 (4.45), 276 (4.25), 332 (4.05) nm; IR νmax (KBr) = 3439, 2920, 1614, 1508, 1452 cm−1; 1H NMR (400 MHz, acetone-d6): δ (ppm) = 1.58 (3H, s, H-25″), 1.71 (3H, s, H-24″), 1.77 (3H, s, H-7″), 2.00 (1H, overlapped, H-6a″), 2.72 (1H, dd, J = 17.0, 4.8 Hz, H-6b″), 2.96-3.00 (1H, m, H-4″), 2.96-3.00 (1H, m, H-5″), 3.32 (1H, d, J = 6.9 Hz, H-21″), 3.63 (1H, br t, J = 5.4 Hz, H-3″), 5.20 (1H, t, J = 6.9 Hz, H-22″), 6.34 (1H, d, J = 8.4 Hz, H-13″), 6.35 (1H, dd, J = 8.4, 2.6 Hz, H-5), 6.36 (1H, d, J = 2.6 Hz, H-17″), 6.41 (1H, d, J = 2.6 Hz, H-3), 6.44 (1H, br d, J = 5.4 Hz, H-2″), 6.51 (1H, dd, J = 8.4, 2.6 Hz, H-19″), 6.63 (1H, br s, H-2′), 6.65 (1H, br s, H-6′), 6.86 (1H, d, J = 16.5 Hz, H-β), 7.12 (1H, d, J = 8.4 Hz, H-14″), 7.15 (1H, d, J = 8.4 Hz, H-20″), 7.30 (1H, d, J = 16.5 Hz, H-α), 7.38 (1H, J = d, 8.4 Hz, H-6); 13C NMR (100 MHz, acetone-d6): δ (ppm) = 17.9 (CH3, C-24″), 23.0 (CH2, C-21″), 23.8 (CH3, C-7″), 25.9 (CH3, C-25″), 28.6 (CH, C-5″), 35.4 (CH, C-3″), 35.9 (CH, C-6″), 37.2 (CH, C-4″), 103.5 (CH, C-3), 103.6 (C, C-8″), 103.9 (CH, C-17″), 106.9 (CH, C-6′), 107.1 (CH, C-13″), 107.5 (CH, C-2′), 108.4 (CH, C-5), 110.2 (CH, C-19″), 111.6 (C, C-4′), 116.9 (C, C-11″), 117.1 (C, C-1), 117.1 (C, C-9″), 117.2 (C, C-15″), 112.8 (CH, C-2″), 123.8 (CH, C-22″), 124.6 (CH, C-α), 125.3 (CH, C-β), 126.1 (CH, C-14″), 127.9 (CH, C-20″), 128.3 (CH, C-6), 131.2 (C, C-23″), 133.5 (C, C-1″), 139.4 (C, C-1′), 152.6 (C, C-16″), 153.3 (C, C-3′), 155.5 (C, C-5′), 156.9 (C, C-10″), 157.5 (C, C-12″), 157.6 (C, C-4), 157.8 (C, C-18″), 159.1 (C, C-2); ESI-MS: m/z 632.5 (C39H36O8). The data were in agreement with the literature.14
Cytotoxicity assay against P388 murine leukemia cell was carried out based on published reports.1516 The leukemia cells at a density of 3 × 104 cell/mL, were plated in 96-well cultures dishes and incubated at 37 ℃ in humidified CO2 incubator (5%) for 24 h. Guangsangon E (1), chalcomoracin (2) and sorocein I (3) were first dissolved in DMSO at the required concentrations and prepared subsequently in six desirable concentrations (100 – 0.1 µg/mL) using PBS (phosphoric buffer solution, pH = 7.30–7.65). The prepared samples were added into the sample wells. Meanwhile, the control wells received only DMSO and PBS. Afterward the cell cultures were incubated again for 48 h in 5% CO2 incubator at 37 ℃. After the incubation, MTT solution [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was added and incubated for 4 h. In the next step, MTT-stop solution containing sodium dodecyl sulphate (SDS) were added, and then the last incubation for 24 h was conducted. Cell survival was evaluated with a multi-well scanning spectrophotometer (ELX 800 UV, universal Microplate Reader, Bio-Tek Instruments, Inc.) at 540 nm. All data are presented as the mean values of triplicates.
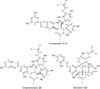
Compound 1 was isolated from fraction C of the hairy root extract of M. macroura as a brown amorphous gum. According to the spectroscopic analysis (NMR and MS) and comparing with the literature,3 compound 1 was assigned to guangsangon E. The molecular structure of guangsangon E (1) is depicted in Fig. 2. Guangsangon E (1) is a Diels-Alder type adduct derived from a substituted chalcone and a substituted 2-phenylbenzofuran. In this work, guangsangon E (1) was tested against P-388 murine leukemia cell line. Compared to the other isolated compounds (2 and 3), guangsangon E (1) exhibited the most potent cytotoxicity against P-388 murine leukemia cell line with IC50 value of 2.75 ± 0.32 µg/mL. To the best of our knowledge, this is the first report concerning cytotoxic activity of guangsangon E (1). Compound 1 was firstly isolated from stem bark of M. macroura and possessed anti-oxidant and anti-inflammation activities.3 Recently, guangsangon E (1) has been reported actively inhibiting tyrosinase.6
Compound 2 was isolated and purified from fraction C as a yellow amorphous gum. NMR and MS spectra analysis and data comparison to references1213 concluded that compound 2 is chalcomoracin, a Diels-Alder type adduct derived from a dehydroprenylchalcone and a substituted 2-arylbenzofuran. The structure of chalcomoracin (2) is depicted in Fig. 2. Chalcomoracin (2) is a phytoalexin, collected for the first time from diseased mulberry leaves (M. àlba).12 Chalcomoracin (2) has been reported having antibiotic activity via inhibition of enoyl-acyl carrier reductase.17
According to the cytotoxic assay, chalcomoracin (2) showed moderate cytotoxicity against P-388 murine leukemia cell line with IC50 value of 5.46 ± 0.55 µg/mL. Chalcomoracin (2) was reported possessing moderate cytotoxic activities against five human cancer cell lines, including A549, Bel 7402, BGC 823, HCT-8, and A2780.18 Recently, molecular mechanism of chalcomoracin (2) as anticancer agent had been reported.19 Chalcomoracin (2) inhibited the cancer cell growth by promoting paraptosis and triggering oxidative stress via a mitophagy.19 Therefore, the result of cytotoxic assay of chalcomoracin (2) against P-388 murine leukemia cell line was in accordance with the previous reports1819 and confirmed chalcomoracin (2) as potent anticancer agent.
Compound 3 was isolated as a purple amorphous gum. The structure of compound 3 was determined based on NMR and MS data analysis and spectral comparison to reported work.14 Compound 3 was assigned as sorocein I, a ketalized Diels-Alder type adduct formed from a dehydroprenylchalcone and a dehydroprenylstilbene. Sorocein I (1) was isolated for the first time from Sorocea ilicifolia.14 Molecular structure of sorocein I (3) was depicted in Fig. 2. Cytotoxic assay revealed that sorocein I (3) was not active against P-388 murine leukemia cell line.
In this report, three Diels-Alder type adducts, guangsangon E (1), chalcomoracin (2) and sorocein I (3) were isolated from hairy root cultures of M. macroura. Guangsangon E (1) and sorocein I (3) were recorded for the first time in this tissue culture. Furthermore, cytotoxic activities of guangsangon E (1) and sorocein I (3) were reported for the first time as well.
Figures and Tables
Acknowledgments
This study was partially funded by Directorate General of Higher Education under research contract No. 127/SP2H/PTNBH/DRPM/2018. We are grateful to Dr. Jalifah Latif, UKM, Malaysia, for the NMR measurements.
References
1. Heyne K. Research and Development Agency. Ministry of Forestry. Indonesia: 1987. p. 659.
2. Dai SJ, Ma ZB, Wu Y, Chen RY, Yu DQ. Phytochemistry. 2004; 65:3135–3141.
3. Dai SJ, Mi ZM, Ma ZB, Li S, Chen RY, Yu DQ. Planta Med. 2004; 70:758–763.
4. Dai SJ, Wu Y, Wang YH, He WY, Chen RY, Yu DQ. Chem Pharm Bull. 2004; 52:1190–1193.
5. Dai SJ, Yu DQ. Nat Prod Res. 2006; 20:676–679.
6. Wang YF, Xu L, Gao W, Niu L, Huang C, Yang P, Hu X. Planta Med. 2018; 84:336–343.
7. Wang YF, Yu MH, Xu L, Niu L, Huang C, Xu H, Yang P, Hu X. Phytochem Lett. 2018; 26:149–153.
8. Sun SG, Chen RY, Yu DQ. J Asian Nat Prod Res. 2001; 3:253–259.
9. Syah YM, Achmad SA, Ghisalberti EL, Hakim EH, Iman MZ, Makmur L, Mujahiddin D. Fitoterapia. 2000; 71:630–635.
10. Syah YM, Achmad SA, Ghisalberti EL, Hakim EH, Makmur L, Soekamto NH. J Chem Res. 2004; 2004:339–340.
11. Happyana N, Mujahiddin D, Juliawaty LD, Makmur L, Achmad SA, Syah YM, Hakim EH. Proceeding of The International Seminar on Chemistry. Indonesia: Universitas Padjadjaran;2008. p. 257–261.
12. Kang J, Chen RY, Yu DQ. Planta Med. 2006; 72:52–59.
13. Takasugi M, Nagao S, Masamune T, Shirata A, Takahashi K. Chem Lett. 1980; 9:1573–1576.
14. Ferrari F, Delle Monache F. Fitoterapia. 2001; 72:301–303.
15. Sahidin Hakim EH, Juliawaty LD, Syah YM, bin Din L, Ghisalberti EL, Latip J, Said IM, Achmad SA. Z Naturforsch C. 2005; 60:723–727.
16. Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Cancer Res. 1988; 48:589–601.
17. Kim YJ, Sohn MJ, Kim WG. Biol Pharm Bull. 2012; 35:791–795.
18. Zhang QJ, Tang YB, Chen RY, Yu DQ. Chem Biodivers. 2007; 4:1533–1540.
19. Han H, Chou CC, Li R, Liu J, Zhang L, Zhu W, Hu J, Yang B. Tian J Sci Rep. 2018; 8:9566.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download