Abstract
Albizzia julibrissin (AJ) is an herbal medicine that shows low toxicity, promotes promoting blood circulation and mitigates the inflammation and has mild side effects. Benign prostate hyperplasia (BPH) is one of the most common diseases that occurs in older males and often results in lower urinary tract symptoms. This study was conducted to evaluate the protective effect of AJ against BPH using LNCaP cells and Sprague Dawley rats treated with testosterone. Treatment with AJ extract reduced the expression of androgen receptor (AR) and prostate-specific antigen (PSA) in vitro. In vivo, rats were divided into 6 groups: 1 (Normal Control); 2 (Testosterone propionate (TP) alone); 3 (TP + finasteride); 4 (TP + AJ 10 mg/kg); 5 (TP + AJ 50 mg/kg); 6 (TP + AJ 300 mg/kg). The groups treated with AJ showed reduced the relative prostate weights and BPH-related proteins were altered, with decreased AR, PSA and proliferating cell nuclear antigen (PCNA) observed by western blot. Histopathological analysis revealed the therapeutic effect of AJ, with a decreased thickness of epithelial cells and reduced level of PCNA and 5α-reductase type 2. These results suggest that AJ extract could ameliorate testosterone-induced benign prostatic hyperplasia.
Dried Albizzia julibrissin (AJ), which is a species of tree in the family Mimosaceae, is used as an herbal medicine to cure bruises, ulcers, abscesses, boils, haemorrhoids and fractures,1 having mild side effects and low toxicity. Saponins, lignans, phenolic glycosides, triterpenes and flavonoids have been identified as the major constituents of the bark of AJ2 that was studied for excellent antioxidant, antidiabetic and anti-tumoral activity.345 It is traditionally administered to help maintain a balanced of mind and promote blood circulation. AJ has an antidepressant-like effects that involve the 5HT1A receptor system.6 Its anti-angiogenic effect on reproductive system have also been demonstrated.7 Additionally, AJ exhibits antioxidant, anticancer and anti-inflammatory properties.8
Benign prostate hyperplasia(BPH), defined as the proliferation of smooth muscle and epithelial cells in the transitional zone of prostate,9 is one of the most common diseases occurring in older males, including in more than 80% of men aged 70 to 79 years of age.10 BPH causes gland enlargement and often results in lower urinary tract symptoms (LUTS) including frequent urination, urgency incontinence and nocturia; these symptoms are associated with increased risk of obstruction, urinary retention, and urinary infection.11 The pathogenesis of BPH is also associated with prostate inflammation.1213
Although the Precise Molecular Mechanisms Underlying The Induction, Maintenance, And Development Of Clinical Sequelae Resulting From Bph Are Not Completely Understood, Androgen And Testosterone Related Hormones Are Considered As The Major Contributing Factors.14 Dihydrotestosterone (Dht) Is A Potent Androgen Synthesized From Testosterone By 5α-reductase Isoenzyme Type 1,2, And 3.1516 Type 2 5α-reductase, Which Is The Main Enzyme Involved In Dht Synthesis, Is Dominant Isoenzyme In Androgen Target Organs Including The Genital Skin, Prostate And Seminal Vesicle.17 Upon Binding To Dht, The Androgen Receptor Translocates To The Nucleus, Binds To Its Target Genes And Regulates Their Expression.18 The Gene And Protein Expression Of Androgen And Estrogen Receptors Are Increased With Increasing In Prostate Size In Patients With Bph.19 Stimulation Of The Androgen Receptor (Ar) By Increased Androgen Levels, Particularly Dht, Induces The Growth Of Prostate Epithelial And Stromal Cells, Resulting In An Enlarged Prostate Transition Zone.20 Proliferating Cell Nuclear Antigen (Pcna) Is An Important Factor In Cell Proliferation, And Increased Pcna Has Been Reported In Testosterone Propionate (Tp)-induced Bph.21 Benign Prostate Hyperplastic Tissue Produces Prostate-specific Antigen (Psa).22 Because High Concentrations Of Dht Enhances Psa Levels By Binding To Ar, Psa Is Widely Used To Help In The Diagnosis Of Bph.2324
Although many pharmacological effects of the AJ have been demonstrated the pharmacological effects of AJ extract on testosterone-induced prostatic hyperplasia have not been studied.In this study, we examined the inhibitory effects of an extract of Albizzia julibrissin on the testosterone-induced rat model by measuring changes in the prostate weight and expression of DHT, 5α-reductase, and PCNA as well as prostate histomorphology. Our results indicate that AJ is helpful for preventing prostatic hyperplasia.
The herbAJ was purchased from Dongkyoung General Trading Company (Dandong, China) and obtained from by Prof. Gyu Yong Son, Chungnam University College of Medicine (Daejeon, Korea). LNCaP cell was form American Type Culture Collection (Manassas, VA, USA). Testosterone propionate was from Tokyo Chemical Industry Co. (Tokyo, Japan). Anti-prostate-specific antigen was form Bioworld Technology (St.Louis Park, MN, USA). The polyvinylidene fluoride membrane transfer system was from Bio-Rad (Hercules, CA, USA). Anti-AR, anti-5α-reductase and peroxidase-conjugated donkey anti-goat Ig G were from Santa Cruz Biotechnology (Dallus, TX, USA). Anti-PCNA and β-actin were from Abcam (Cambridge, UK). The enhanced chemiluminescence detection (ECL) kit was obtained from Amersham Pharmacia Biotech (Amersham, UK).
Ground AJ (20 g) was extracted in 300 mL methanol at 40 ℃ for 3 h. The solution was filtered through filter paper, and the residue was extracted in 300 mL methanol at 40 ℃ for 3 h. Filtration was repeated 2 more times. After filtration, the solution was collected, and then concentrated under reduced pressure, and 1.67 g of AJextract (HHP) was obtained. Stock solutions were stored in a freezer.
Androgen-dependent human prostate adenocarcinoma cells of the LNCaP cell line were cultured in RPMI 1640 (Gibco, Grand Island, NY, USA) medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 ℃ in a 5% CO2 atmosphere. The medium was replaced every 48 h.
LNCaP cells were seeded into 6-well plates (5 × 105 cells/well) in 1 mL of RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37 ℃ for 15 h. Testosterone (100 nM) and 100 µg/mL HHP (0, 10, 50, 300 µg/mL) were added to the cells, and the cells were collected after culture for 72 h.
For all assays, LNCaP cells were seeded at a density of 1 × 104 cells/mL in 96-well plates with regular growth medium (RPMI 1640 supplemented with FBS and antibiotics) and experiments were carried out on the following day. The effect of HHP on LNCaP cells was assessed by MTT assays. LNCaP cells were treated with the HHP (0, 10, 50, 300 µg/mL) and incubated for 72 h. The MTT assay was performed using the EZ-Cytox Cell Viability Assay Kit (Daeil Lab Service, Seoul, Korea) according to the manufacturer's protocol.
All animal experimental protocols were approved by the institutional animal ethics committee at Chungnam National University.(CNU-00446) Six-week-old healthy male Sprague-Dawley rats were obtained from Orient Bio (Seongnam, Korea). All rats were housed in a pathogen-free animal facility at Chungnam National University with a 12 hours light/dark cycle at 20 – 25 ℃ under the 30 – 35% relative humidity condition and were freely provided with food (Orient Bio) and water. After 7 days of acclimatization, all rats were subcutaneously administered testosterone propionate (3 mg/kg, Tokyo Chemical Ins. Co., Tokyo, Japan) with co-treatment of AJ orally for 28 consecutive days, except for rats in the control groups (n = 7 rats/group). The following groups were used: group 1 (normal control, NC), group 2 (testosterone alone, BPH), group 3 (Fina; BPH+finasteride), group 4 (HHP-10; BPH+HHP 10 mg/kg), group 5 (HHP-50; BPH+HHP 50 mg/kg), and group 6 (HHP-300; BPH+ HHP 300 mg/kg). After the final treatment, all rats were anesthetized with carbon dioxide and sacrificed. The prostate was carefully dissected, weighed, and processed for western blot, histopathological, and immunohistochemistry analyses.
Collected cells and frozen prostate tissue samples were homogenized and lysed in RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium dodecyl sulfate, protease inhibitor cocktail). The homogenate was centrifuged (12,000 rpm for 20 min at 4 ℃) and the supernatant was analyzed by western blotting. After determining the protein concentration by using the BCA assay, 30 µg of protein was separated by electrophoresis using 8 – 12% sodium dodecyl sulfate gels. Proteins were electrotransferred onto polyvinylidene fluoride membranes in a Semi-Dry Transfer System. The membrane was blocked with 5% skim milk and incubated with the following primary antibodies: anti-PCNA (1:3000, Abcam), anti-androgen receptor (AR, 1:1000, Santa Cruz), anti-PSA (1:1000, Bioworld Technology), and β-actin (1:1000, Abcam). The membranes were washed in PBS-T buffer and incubated with peroxidase-conjugated goat anti-rabbit (1:5000, AbFrontier, Seoul, Korea) or donkey anti-goat (1:5000, Santa Cruz) IgG antibody for 1 hours. Proteins were visualized with an enhanced chemiluminescence detection kit (Amersham).
The prostate was fixed immediately in 10% buffered formalin phosphate solution, embedded in paraffin, and cut into 5-µm sections and processed for histological staining. These serial tissue sections were either stained with hematoxylin and eosin for histological examination or subjected to immunohistochemistry staining.
Immunohistochemical analysis was performed using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. After antigen retrieval and processing, the sections were blocked in normal serum and then incubated with anti-PCNA (Abcam) antibody and anti-5α-reductase 2 (1:100; Santa Cruz) overnight. The sections were incubated with the Vectastain Elite ABC goat or mouse IgG kit according to the manufacturer's protocol, and the 3, 3′-diaminobenzidine was added for color development. Mayer's hematoxylin was used for counterstaining. All stained sections were evaluated in 10 randomly selected fields under a light microscope (Eclipse 80; Nikon, Tokyo, Japan).
The results were expressed as the means ± standard deviation (SD). Statistical differences were determined by one-way analysis of variance or nonparametric Kruskal-Wallis test, followed by post-hoc analysis using Tukey's multiple comparison test. P values less than 0.05 were considered statistically significant.
BPH is associated with androgens, and thus, may have curative and preventive effects or alleviate symptoms by inhibiting the expression of androgen receptors. Many studies have focused on modulating the targets of the 5α-reductase-AR pathway that contribute to hyperplasia of the prostate25 In this study, the group treated with testosterone alone showed proliferation of LNCaP cells and histopathological alterations with BPH.
Herbal medicines have few side effects and show therapeutic effects. In this study, the effects of AJ treatment on LNCaP cells and testosterone-induced BPH were investigated. The cell viability assay revealed cell toxicity at a concentration of 1000 µg/mL. The effects of finasteride, a type 2 5α-reductase inhibitor, were compared with those of AJ. The expression of AR and PSA was enhanced after testosterone treatment. Finasteride cotreatment successfully suppressed AR and PSA expression in cells treated with testosterone. The AR signal was decreased when 100 µg/mL AJ was used to treat the cells compared to LNCaP cells treated with testosterone alone (Fig. 1a). Furthermore, AJ reduced PSA (Fig. 1b). AJ showed concentration-dependent therapeutic effects on BPH.
The effects of AJ treatment on testosterone-induced BPH were examined in an in vivo animal study. After 28 consecutive days of testosterone treatment, the prostate weight was increased. In the group treated with testosterone alone, the relative prostate weights were increased by 2-fold compared to those in control rats. The relative weights of the prostate treated with AJ were significantly decreased compared to those in the group treated with testosterone alone, but the effects were not concentrationdependent (Table 1). Morphologically, dramatic histopathological changes in the ventral prostate were observed after testosterone treatment compared to those in the control groups; particularly, the thickness of the prostate epithelial was compared. In all groups treated with finasteride and AJ, the prostate epithelial thickness was significantly decreased. Thus, AJ reduced BPH, particularly by affecting epithelial cells (Fig. 2).
BPH-related protein expression patterns were investigated in the prostate tissue by western blot and histopathological analyses. Testosterone treatment significantly enhanced AR and PSA expression. The expression of AR, which binds to DHT, was decreased in the groups treated with AJ; particularly, in the 300 mg/kg AJ treatment group. AR expression was significantly decreased compared to that in the lower concentrations. The PSA level was also significantly decreased in both groups treated with 50 and 300 mg/kg, but not in groups treated with 10 mg/kg (Fig. 3). Thus, AJ significantly downregulated the level of AR and PSA. Immunohistochemistry staining for 5α-reductase type 2 supported these results. 5α-Reductase type 2 was mainly expressed in the cytoplasm of epithelial cells. The area of 5α-reductase-stained cells was significantly increased in the testosterone-treated groups. In the groups treated with AJ, the area of 5α-reductase staining was significantly decreased in a concentration-dependent manner (Fig. 4). This indicated that 5α-reductase-AR is the main pathway involved in BPH, and AJ can be used as a pharmaceutical therapy for treating BPH.
PCNA is an acidic nuclear protein expressed mainly in the S phase of the cellular cycle, It shows increased expression in prostate cancer and BPH tissues compared to that in the normal prostate tissue.26 According to western blot analysis, PCNA expression was significantly decreased in both groups treated with AJ 50 and 300 mg/kg compared to that in groups treated with testosterone alone (Fig. 5a and 5b). Immunohistochemistry staining also showed that the number of PCNA-stained cell nuclei of epithelial cells was significantly decreased in the groups treated with AJ in a concentration-dependent manner (Fig. 5c and 5d). These results show that AJ decreased the S phase of the cellular cycle, and thus had a therapeutic effect on BPH.
In this study, we evaluated the effect of AJ on BPH in vitro and in vivo. Treatment with TP increased the relative prostate weight as well as the levels of AR, PSA, PCNA, and 5α-reductase. However, in the AJ-treated model, AR, PSA, PCNA, and 5α-reductase levels were reduced as assessed by western blot analysis and immunohistochemical staining of the prostate tissue.
The main treatments for enlarged prostate are α-blockers and 5α-reductase inhibitor.9 Alpha-blockers are effective in alleviating LUTS across a wide spectrum of baseline parameters.27 While 5α-reductase inhibitor decreases the size of the prostate and slows the prostate growth.23 Its mechanism of action is that by inhibiting the conversion from testosterone to DHT, the level of circulating DHT is reduced. However, the side effects of 5α-reductase inhibitors include erectile dysfunction, ejaculatory dysfunction, decreased libido, and rarely, gynecomastia.27 Therefore, the interest about the herbal medicine is increased for the alternative therapy. Because AJ has various properties, such as anti-inflammation and antioxidant,8 it is less toxic than chemical drugs. In this study, as we mentioned earlier, AJ reduced the prostate's weights and suppressed the level of BPH-related protein, AR, PSA, PCA and 5α-reductase, AJ can be used as a therapeutic agent for BPH. When to consider the paper that saponin and phenolics have anti-proliferative effect on LNCaP cell and inhibitory effect against 5α-reductase,2930 we suggest that saponin and phenolic glycosides may be potential chemical constituents for effect of mitigating the BPH. However, further investigations on this effect are needed to evaluate the detail for further study.
Figures and Tables
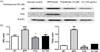 | Fig. 1Effect of AJ extracts on expression of PSA and AR via western blot in vitro.(a) Cells were incubated with (NC)/without testosterone (100 nM) alone (BPH) or testosterone with finasteride (10 µM) or testosterone with AJ (100 µg/mL). The expression of AR and PSA was reduced compared to that in the group treated with testosterone alone. (b) Band intensities were normalized to that of β-actin. Values are expressed as the means ± standard deviation (SD). *p < 0.05 indicates significant difference and **p < 0.01 indicates significant difference compared to the BPH alone group ###p < 0.001 indicates significant difference compared to the control group. ##p < 0.01 indicates significant difference compared to the control group.
|
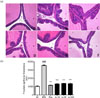 | Fig. 2Effects of AJ on prostate histopathology determined by hematoxylin and eosin staining of tissue.Magnification ×400.(A) Control; (B) BPH; (C) Fina; BPH+finasteride (5 mg/kg P.O.) (D) AJ-10; BPH+AJ (10 mg/kg P.O.); (E) AJ-50; BPH+AJ (50 mg/kg P.O.); (F) AJ-300; BPH+AJ (300 mg/kg P.O.); treated group. To induce BPH, all rats were administered TP (3 mg/kg, S.C.) for 28 consecutive days, except for the N/C (vehicle injected). Prostate epithelial thickness was measured with an image analyzer. ###p < 0.001 indicates a significant difference compared to the N/C group, ***p < 0.001 indicates a significant difference compared to the BPH alone group.
|
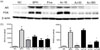 | Fig. 3Effect of AJ extracts on expression of PSA and AR protein by western blotting in vivo.(a) Cells were incubated with (NC)/without testosterone (100 nM) alone (BPH) or testosterone with finasteride (10 µM) or testosterone with AJ (100 µg/mL). The expression of AR and PSA was reduced compared to in the group treated with testosterone alone. (b) Band intensities were normalized to that of β-actin. Values are expressed as the means ± standard deviation (SD). *p < 0.05 indicates significant difference and **p < 0.01 indicates significant difference compared to the BPH alone group ###p < 0.001 indicates significant difference compared to the control group. ##p < 0.01 indicates significant difference compared to the control group.
|
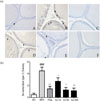 | Fig. 4Expression 5α-reductase by immunohistochemistry in each group.Magnification ×400. (a) Immunohistochemistry analysis of expression of 5α-reductase type 2 in prostate groups. (b) 5α-Reductase type 2 positive cells were expressed as each positive area (% area) A; NC, B: BPH; C: Fina; BPH+finasteride (5 mg/kg P.O.); (D) AJ-10; BPH+AJ (10 mg/kg P.O.); (E) AJ-50; BPH+AJ (50 mg/kg P.O.); (F) AJ-300; BPH+AJ (300 mg/kg P.O.); treated group. To induce BPH, all rats were administered TP (3 mg/kg, S.C.) for 28 consecutive days, except for the N/C (vehicle injected). Arrow indicates 5α-reductase type 2-positive stained prostate epithelial cells. ###p < 0.001 indicates a significant difference compared to the N/C group, **p < 0.01 indicates a significant difference compared to the BPH alone group. *p < 0.5 indicates significant difference compared to the BPH group treated with testosterone alone.
|
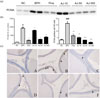 | Fig. 5Expression PCNA of the prostate tissue in each group.Magnification ×400. (a) Expression of PCNA using western blot analysis (b) Band intensities were assessed by densitometry and normalized to that of β-actin. (c) Immunohistochemistry analysis of expression of PCNA in prostate. (d) PCNA-positive cells were expressed as each positive cell/100 cells. A; NC, B: BPH; C: Fina; BPH+finasteride (5 mg/kg P.O.); (D) AJ-10; BPH+AJ (10 mg/kg P.O.); (E) AJ-50; BPH+AJ (50 mg/kg P.O.); (F) AJ-300; BPH+AJ (300 mg/kg P.O.); treated group. To induce BPH, all rats were administered TP (3 mg/kg, S.C.) for 28 consecutive days, except for the N/C (vehicle injected). Arrow indicates PCNA-positive stained prostate epithelial cells. ###p < 0.001 indicates a significant difference compared to the N/C group, **p < 0.01 indicates a significant difference compared to the BPH alone group. ***p < 0.001 indicates a significant difference compared to the BPH group.
|
Table 1
Effect of AJ extracts on body weight and prostate weights.

(A) Control; (B) BPH; (C) Fina; BPH+finasteride (5 mg/kg P.O.) (D) AJ-10; BPH+AJ (10 mg/kg P.O.); (E) AJ-50; BPH+AJ (50 mg/kg P.O.); (F) AJ-300; BPH+AJ (300 mg/kg P.O.); treated group. To induce BPH, all rats were administered TP (3 mg/kg, S.C.) for 28 consecutive days, except for the N/C (vehicle injected). Data are presented as the means ± SD (n=5). **p < 0.01 indicates significant difference compared to the BPH alone group. ***p < 0.01 indicates significant difference compared to the BPH alone group. ###p < 0.001 indicates significant difference compared to the control group.
References
1. Higichi H, Kinjo J, Nohara T. Chem Pharm Bull (Tokyo). 1992; 40:829–831.
2. Lv JS, Zhang LN, Song YZ, Wang XF, Chu XZ. Int Biodeterior Biodegrad. 2011; 65:258–264.
3. Lau CS, Carrier DJ, Beitle RR, Bransby DI, Howard LR, Lay JO Jr, Liyanage R, Clausen EC. Bioresour Technol. 2007; 98:429–435.
4. Zheng L, Zheng J, Zhao Y, Wang B, Wu L, Liang H. Bioorg Med Chem Lett. 2006; 16:2765–2768.
5. Kang TH, Jeong SJ, Kim NY, Higuchi R, Kim YC. J Ethnopharmacol. 2000; 71:321–323.
6. Kim JH, Kim SY, Lee SY, Jang CG. Pharmacol Biochem Behav. 2007; 87:41–47.
7. Gupta RS, Chaudhary R, Yadav RK, Verma SK, Dobhal MP. J Ethnopharmacol. 2005; 96:31–36.
8. Kokila K, Priyadharshini SD, Sujatha V. Int J Pharm Pharm Sci. 2013; 5:70–73.
9. Priest R, Garzotto M, Kaufman J. Tech Vasc Interv Radiol. 2012; 15:261–264.
11. Miller J, Tarter TH. Clin Interv Aging. 2009; 4:251–258.
12. Nickel JC. Urol Clin North Am. 2008; 35:109–115.
14. Choi HM, Jung Y, Park J, Kim HL, Youn DH, Kang JW, Jeong MY, Lee JH, Yang WM, Lee SG, Ahn KS, Um JY. Sci Rep. 2016; 6:31906.
15. Azzouni F, Godoy A, Li Y, Mohler J. Adv Urol. 2012; 2012:530121.
16. Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D. J Urol. 2004; 172:1399–1403.
17. Goldenberg L, So A, Fleshner N, Rendon R, Drachenberg D, Elhilali M. Can Urol Assoc J. 2009; 3:S109–S114.
18. Lonergan PE, Tindall DJ. J Carcinog. 2011; 10:20.
19. Sreenivasulu K, Nandeesha H, Dorairajn LN, Rajesh NG. Indian J Pathol Microbiol. 2019; 62:99–102.
20. Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Am J Pathol. 2013; 182:1942–1949.
21. Bae JS, Park HS, Park JW, Li SH, Chun YS. J Nat Med. 2012; 66:476–485.
22. Lepor H. Rev Urol. 2004; 6 Suppl 9:S3–S10.
23. Velonas VM, Woo HH, dos Remedios CG, Assinder SJ. Int J Mol Sci. 2013; 14:11034–11060.
24. Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somervile MC, Rittmaster RS. Eur Urol. 2008; 54:1379–1384.
25. Schmidt LJ, Tindall DJ. J Steroid Biochem Mol Biol. 2011; 125:32–38.
26. Zhong WD, Peng J, He HC, Wu D, Han ZD, Bi XC, Dai QS. Clin Invest Med. 2008; 31:E8–E15.
27. Kapoor A. Can J Urol. 2012; 19:10–17.
28. Djavan B, Marberger M. T. Eur Urol. 1999; 36:1–13.
29. Liu WK, Xu SX, Che CT. Life Sci. 2000; 67:1297–1306.
30. Hussein SA, Hashim AN, Barakat HH, Jose J, Lindequist U, Nawwar MA. Pharmazie. 2006; 61:1034–1037.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download