Abstract
Osteoporosis is a skeletal disorder characterized by compromised bone strength resulting in a predisposition to fracture. Osteoporosis-related fractures can lead to pain, disability, and increased healthcare costs. This study aimed to explore different pharmacological treatments for osteoporosis. Various treatments are used to prevent and treat osteoporosis, particularly in postmenopausal women and elderly men, but the approach needs to be individually tailored. Bisphosphonates are most commonly used to treat osteoporosis. Bisphosphonates and denosumab are mainly used during the initial phase of therapy for most patients with osteoporosis, including those with a high risk of fracture. In younger postmenopausal women, menopausal hormone therapy (including tibolone) and selective estrogen receptor modulators may be considered as alternatives for fracture prevention. Parathyroid hormone therapy is recommended for osteoporosis treatment in elderly patients with an increased risk of multiple vertebral fractures. Dual energy X-ray absorptiometry (DXA) is the mainstay for monitoring the treatment response, and clinicians may consider alternative treatments if a significant decrease in bone mineral density is detected (using DXA or bone turnover markers) or if recurrent fractures occur during treatment. For postmenopausal women undergoing long-term bisphosphonate treatment, the risk of fracture should be reassessed after 3 to 5 years, and a “drug holiday” should be considered if the risk of fracture is low-to-moderate. Therapy should be continued for patients who continue to exhibit a high risk of fracture, or alternatively, switching to other treatments may be considered.
Figures and Tables
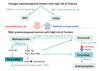
 | Figure 1Algorithm for the drug holiday period during the long-term bisphosphonate therapy in postmenopausal women. IV, intravennous; BMD, bone mineral density. Reproduced from Korean Society for Bone and Mineral Resaerch. Physician's guide for diagnosis and treatment of osteoporosis 2015 [Internet]. Seoul: Korean Society for Bone and Mineral Research; 2015, according to the Creative Commons license [3]. |
References
1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285:785–795.
2. Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002; 23:279–302.

3. Korean Society for Bone and Mineral Resaerch. Physician's guide for diagnosis and treatment of osteoporosis 2015 [Internet]. Seoul: Korean Society for Bone and Mineral Research;2015. cited 2019 Sep 5. Available from: http://www.ksbmr.org/journal/index4.php.
4. Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, Almasri J, Farah W, Sarigianni M, Muthusamy K, Al Nofal A, Haydour Q, Wang Z, Murad MH. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019; 104:1623–1630.

5. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2019; 104:1595–1622.

6. Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD, Narula HS, Pessah-Pollack R, Tangpricha V, Wimalawansa SJ, Watts NB. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis 2016. Endocr Pract. 2016; 22:Suppl 4. 1–42.

7. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004; 291:1701–1712.
8. Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA, Ko MG, Naughton MJ, Satterfield S, Bassford T. Women's Health Initiative Investigators. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women's Health Initiative Randomized Trial. J Bone Miner Res. 2006; 21:817–828.

9. Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Women's Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003; 290:1729–1738.

10. Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, Bluhm E, Connelly S, Hubbell FA, Lane D, Martin L, Ockene J, Rohan T, Schenken R, Wactawski-Wende J. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012; 13:476–486.

12. Delmas PD, Davis SR, Hensen J, Adami S, van Os S, Nijland EA. Effects of tibolone and raloxifene on bone mineral density in osteopenic postmenopausal women. Osteoporos Int. 2008; 19:1153–1160.

13. Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, Kloosterboer L, Mosca L, Christiansen C, Bilezikian J, Kerzberg EM, Johnson S, Zanchetta J, Grobbee DE, Seifert W, Eastell R. LIFT Trial Investigators. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008; 359:697–708.

14. Formoso G, Perrone E, Maltoni S, Balduzzi S, Wilkinson J, Basevi V, Marata AM, Magrini N, D'Amico R, Bassi C, Maestri E. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev. 2016; 10:CD008536.

15. Huang KE, Baber R. Asia Pacific Tibolone Consensus Group. Updated clinical recommendations for the use of tibolone in Asian women. Climacteric. 2010; 13:317–327.

16. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Glüer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999; 282:637–645.

17. Siris ES, Harris ST, Eastell R, Zanchetta JR, Goemaere S, Diez-Perez A, Stock JL, Song J, Qu Y, Kulkarni PM, Siddhanti SR, Wong M, Cummings SR. Continuing Outcomes Relevant to Evista (CORE) Investigators. CORE) Investigators. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. J Bone Miner Res. 2005; 20:1514–1524.

18. Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, Secrest RJ, Cummings SR. CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004; 96:1751–1761.

19. Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, Secrest RJ, Cummings SR. CORE Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006; 355:125–137.

20. Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, Constantine GD, Chines AA. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008; 23:1923–1934.

21. Silverman SL, Chines AA, Kendler DL, Kung AW, Teglbjærg CS, Felsenberg D, Mairon N, Constantine GD, Adachi JD. Bazedoxifene Study Group. Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. Osteoporos Int. 2012; 23:351–363.

22. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009; 92:1045–1052.

23. Pinkerton JV, Harvey JA, Lindsay R, Pan K, Chines AA, Mirkin S, Archer DF. SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014; 99:E189–E198.

24. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361:756–765.

25. Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007; 40:1434–1446.

26. Zhang L, Pang Y, Shi Y, Xu M, Xu X, Zhang J, Ji L, Zhao D. Indirect comparison of teriparatide, denosumab, and oral bisphosphonates for the prevention of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. Menopause. 2015; 22:1021–1025.

27. Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SM, Finkelstein JS. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009; 94:2915–2921.

28. Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016; 375:1532–1543.

29. Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001; 12:989–995.

30. Baber RJ, Panay N, Fenton A. IMS Writing Group. 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. 2016; 19:109–150.

31. Diez-Perez A, Gonzalez-Macias J. Inadequate responders to osteoporosis treatment: proposal for an operational definition. Osteoporos Int. 2008; 19:1511–1516.

32. Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, Blackwell T, Eckert S, Black D. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA. 2000; 283:1318–1321.

33. Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, Eastell R, Eriksen EF, Gonzalez-Macias J, Liberman UA, Wahl DA, Seeman E, Kanis JA, Cooper C. IOF CSA Inadequate Responders Working Group. Treatment failure in osteoporosis. Osteoporos Int. 2012; 23:2769–2774.

34. Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SA. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015; 386:1147–1155.

35. Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, McKinney R Jr, Pignolo RJ, Sellmeyer DE. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016; 31:16–35.

36. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Torring O, Valter I, Wang AT, Brown JP. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018; 33:190–198.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download