Abstract
Objectives
To compare surgical outcomes such as the ambulatory period and survival according to different surgical excision tactics for metastatic spine tumors (MSTs).
Summary of Literature Review
Surgical outcomes, such as pain relief and survival, in patients with MSTs have been reported in several studies, but the effects of differences in surgical extent on the ambulatory period have rarely been reported.
Materials and Methods
Ninety-six patients with MSTs who underwent palliative (n=60) or extensive wide excision (n=36) were included. Palliative excision was defined as partial removal of the tumor as an intralesional piecemeal procedure for decompression. Extensive wide excision was defined as a surgical attempt to remove the whole tumor at the index level as completely as possible. The primary outcome was the ambulatory period following surgery. Other demographic and radiographic parameters were analyzed to identify the risk factors for loss of ambulatory ability and survival. Perioperative complications were also assessed.
Results
The mean postoperative ambulatory period was longer in the extensive wide excision group (average 14.8 months) than in the palliative excision group (average 11.7 months) (p=0.021). The survival rates were not significantly different between the two surgical excision groups (p=0.680). However, postoperative ambulatory status and major complications within 30 days postoperatively were significant prognostic factors for survival (p=0.003 and p=0.032, respectively).
Conclusions
The extent of surgical excision affected the ambulatory period, and the complication rates were similar, regardless of surgical excision tactics. A proper surgical strategy to achieve postoperative ambulatory ability and to reduce perioperative complications would have a favorable effect on survival.
REFERENCES
1. Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010 Jul; 13(1):E94–108. DOI: 10.3171/2010.3.spine09202.
2. Sohn S, Kim J, Chung CK, et al. A nationwide epidemio-logical study of newly diagnosed spine metastasis in the adult Korean population. Spine J. 2016 Mar; 16(8):E937–45. DOI: 10.1016/j.spinee.2016.03.006.

3. Perrin RG, Laxton AW. Metastatic spine disease: epidemi-ology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am. 2004 Sep; 15(4):E365–73. DOI: 10.1016/j.nec.2004.04.018.

4. Al-Qurainy R, Collis E. Metastatic spinal cord compression: diagnosis and management. BMJ (Clinical research ed). 2016 May; E353:i2539. DOI: 10.1136/bmj.i2539.

5. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet (London, England). 2005 Aug; 366(9486):E643–8. DOI: 10.1016/s0140-6736 (05)66954-1.

6. Choi D, Fox Z, Albert T, et al. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. British journal of neurosurgery. 2016 Feb; 30(3):E337–44. DOI: 10.3109/02688697.2015.1133802.

7. Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited sub-mission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. Journal of neurosurgery Spine. 2008 Mar; 8(3):E271–8. DOI: 10.3171/spi/2008/8/3/271.
8. Kakutani K, Sakai Y, Maeno K, et al. Prospective Cohort Study of Performance Status and Activities of Daily Living After Surgery for Spinal Metastasis. Clin Spine Surg. 2017 Oct; 30(8):E1026–32. DOI: 10.1097/bsd.0000000000000456.

9. Tang Y, Qu J, Wu J, et al. Effect of Surgery on Quality of Life of Patients with Spinal Metastasis from Non-Small-Cell Lung Cancer. The Journal of bone and joint surgery American volume. 2016 Mar; 98(5):396–402. DOI: 10.2106/jbjs.o.00629.

10. Fourney DR, Frangou EM, Ryken TC, et al. Spinal insta-bility neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011 Aug; 29(22):3072–7. DOI: 10.1200/jco.2010.34.3897.

11. Tokuhashi Y, Matsuzaki H, Oda H, at al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005 Oct; 30(19):218691. DOI: 10.1097/01.brs.0000180401.06919.a5.
12. Tomita K, Kawahara N, Kobayashi T, at al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001 Feb; 26(3):298–306. DOI: 10.1097/00007632-200102010-00016.
13. Bakar D, Tanenbaum JE, Phan K, et al. Decompression surgery for spinal metastases: a systematic review. Neurosurg focus. 2016 Aug; 41(2):E2. DOI: 10.3171/2016.6.fo-cus16166.

14. Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for sur-gery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010 Feb; 19(2):215–22. DOI: 10.1007/s00586-009-1252-x.

15. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972; 95(1):87–100. DOI: 10.1093/brain/95.1.87.

16. Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010 Sep; 13(3):324–8. DOI: 10.3171/2010.3.spine09459.

17. Ho JC, Tang C, Deegan BJ, et al. The use of spine ste-reotactic radiosurgery for oligometastatic disease. J Neurosurg Spine. 2016 Aug; 25(2):239–47. DOI: 10.3171/2016.1.spine151166.

18. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life”. JAMA. 2008 Feb; 299(8):937–46. DOI: 10.1001/jama.299.8.937.
19. Chaichana KL, Woodworth GF, Sciubba DM, et al. Pre-dictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008 Mar; 62(3):683–92. discussion 683-92. DOI: 10.1227/01.neu.0000317317.33365.15.

20. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001 Jul; 33(5):337–43. DOI: 10.3109/07853890109002087.
21. de Ruiter GC, Nogarede CO, Wolfs JF, at al. Quality of life after different surgical procedures for the treatment of spinal metastases: results of a single-center prospective case series. Neurosurg focus. 2017 Jan; 42(1):E17. DOI: 10.3171/2016.6.focus16150.
22. Kim DG, Ha JK, Hwang CJ, at al. Is 1-stage Posterior Corpectomy More Favorable Compared to Decompression with Fusion to Control Thoracic Cord Compression by Metastasis? Clin Spine Surg. 2017 Oct; 30(8):350–55. DOI: 10.1097/bsd.0000000000000267.
23. Kim CH, Chung CK, Jahng TA, at al. Resumption of ambulatory status after surgery for nonambulatory patients with epidural spinal metastasis. Spine J. 2011 Oct; 11(11):E1015–23. DOI: 10.1016/j.spinee.2011.09.007.
24. Park SJ, Lee CS, Chung SS. Surgical results of metastatic spinal cord compression (MSCC) from non-small cell lung cancer (NSCLC): analysis of functional outcome, survival time, and complication. Spine J. 2016 Mar; 16(3):322–8. DOI: 10.1016/j.spinee.2015.11.005.

25. Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976). 1997 May; 22(9):1036–44. DOI: 10.1097/00007632-199705010-00020.
Fig. 1.
Distribution of primary solid cancers. The most common primary cancer was lung cancer (n=35, 36.5%), followed by hepatobiliary cancer (n=25, 26.0%).
Fig. 2.
Kaplan-Meier curve of the postoperative ambulatory period according to the extent of surgical excision. Patients in the W group could walk for an average of 14.8±1.5 months after surgery (95% CI, 11.9-17.7 months), and patients in the P group could walk for an average of 11.7±1.7 months after surgery (95% CI, 8.3-15.0 months). Surgical extent significantly affected the postoperative ambulatory period (p=0.021).
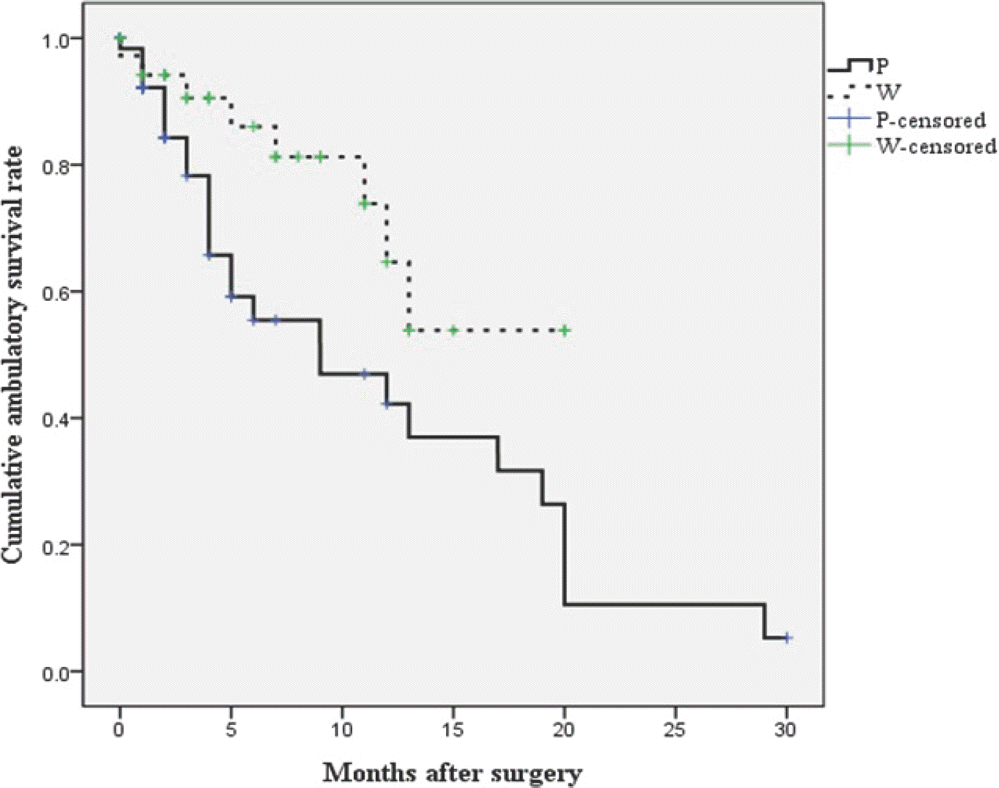
Fig. 3.
Kaplan-Meier curve of the survival rate according to the extent of surgical excision. Surgical extent did not affect the survival rate (p=0.689).
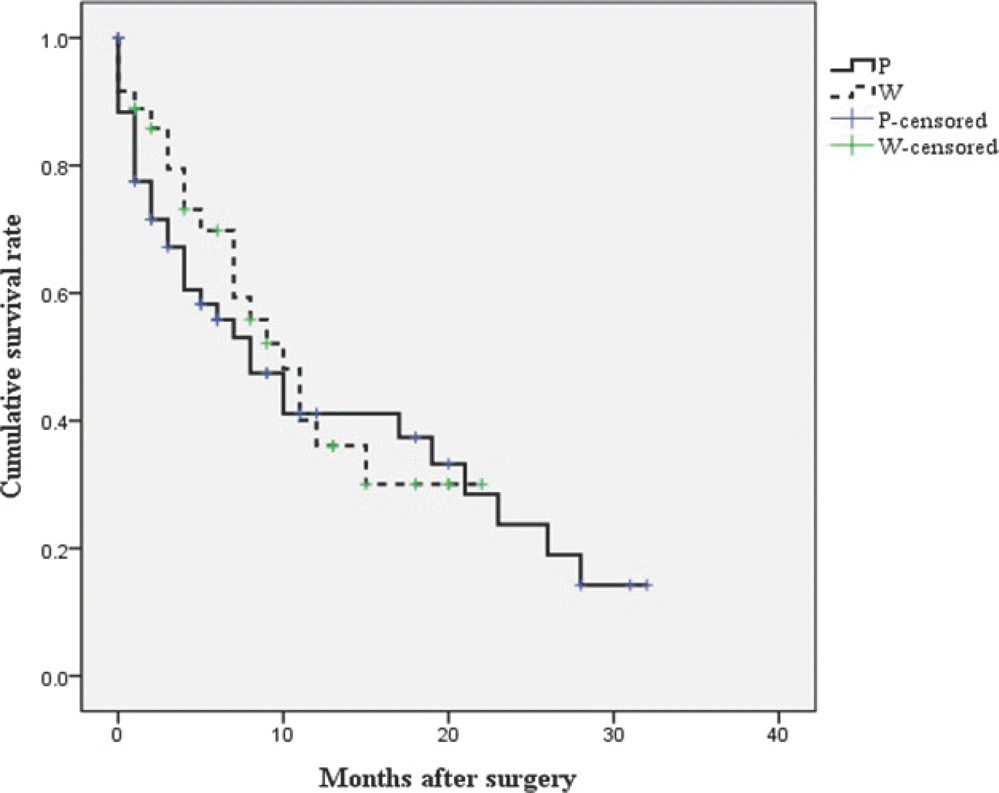
Table 1.
Summary of preoperative patient data
| Total (n=96) | P group (n=60) | W group (n=36) | p | |
|---|---|---|---|---|
| Age (range) | 57.1±12.8(21-86) | 56.2±13.4(21-86) | 58.6±11.6(23-79) | NS∗ |
| Male (n, [%]) | 62(64.6) | 46(76.7) | 16(44.4) | 0.001† |
| Surgical level | ||||
| Cervical | 10 | 4 | 6 | NS‡ |
| Thoracic | 56 | 36 | 20 | |
| Lumbosacral | 30 | 20 | 10 | |
| Tumor diagnosis to surgery, median (month) | 11.0(0-170) | 11.0(0-154) | 12.0(1-170) | NS¶ |
| Symptom to surgery, median (day) | 24.5(0-330) | 21(1-240) | 36.5(0-330) | NS§ |
| Metastasis (n) | ||||
| None | 9 | 6 | 3 | |
| Non-spinal bone | 19 | 15 | 4 | NS‡ |
| Visceral | 37 | 21 | 16 | |
| Both | 31 | 18 | 13 | |
| No. of metastatic spine segments (n) | ||||
| 1-2 | 32 | 17 | 15 | NS† |
| ≥3 | 64 | 43 | 21 | |
| Preop. ECOG-PS (n) | ||||
| 1 | 39 | 22 | 17 | |
| 2 | 25 | 11 | 14 | NS‡ |
| 3 | 25 | 20 | 5 | |
| 4 | 7 | 7 | 0 | |
| Ambulatory (n, [%]) | 73(76.0) | 41(68.3%) | 32(88.9%) | 0.022† |
| ASA physical status classification (n) | ||||
| 1-2 | 75 | 50 | 25 | NS† |
| 3 | 21 | 10 | 11 | |
| Preop. serum albumin (g/dL) | 3.4±0.5 | 3.4±0.5 | 3.6±0.6 | 0.010∗ |
|
≥3.0 (n) <3.0 (n) |
50 10 |
30 6 |
NS† | |
| Preop. embolization (n) | 45 | 19 | 26 | <0.001 |
Table 2.
Epidural cord compression scale on MRI suggested by Bilsky et al.21
| Total (n=96) | P group (n=60) | W group (n=36) | p∗ | |
|---|---|---|---|---|
| 0 | 7(7.3%) | 4(6.7%) | 3(8.3%) | |
| 1 | 19(19.8%) | 11(18.3%) | 8(22.2%) | NS |
| 2 | 19(19.8%) | 10(16.7%) | 9(25%) | |
| 3 | 51 (53.1%) | 35(58.3%) | 16(44.5%) |
Table 3.
Summary about preoperative and postoperative chemotherapy radiotherapy
| P group (n=60) | W group (n=36) | p∗ | |
|---|---|---|---|
| Postop. RT | 33(55%) | 27(75%) | NS |
| Preop. CT | 32(53.3%) | 19(52.8%) | |
| PD | 23 | 15 | NS |
| SD or PR | 9 | 4 | |
| Postop. CT | 27(45%) | 21(58.3%) | NS |
Table 4.
Risk factor analysis for ambulatory outcome
| Loss of ambulatory status during follow-up | Survival∗ | |||||
|---|---|---|---|---|---|---|
| P for univariate analysis | P for multivariate analysis | HR (95% CI) | P for univariate analysis | P for multivariate analysis | HR (95% CI) | |
| Sex | 0.288 | 0.886 | ||||
| Age (≥60 vs. <60 y) | 0.128 | 0.589 | 0.740 | |||
| Location of metastasis | 0.407 | 0.911 | ||||
| Wide excision vs. Palliative excision | 0.030 | 0.034 | 0.418(0.187-0.935) | 0.689 | ||
| Adjuvant RT (Yes vs. No) | 0.441 | 0.007 | 0.191 | |||
| Preop. CT (No vs. PD or SD/PR) | 0.056 | 0.170 | 0.362 | |||
| Postop. CT (Yes vs. No) | 0.333 | 0.486 | ||||
| Preop. ECOG-PS (1-2 vs. 3-4) | 0.583 | 0.002 | 0.499 | |||
| Preop. ambulatory (Yes vs. No) | 0.264 | <0.001 | 0.951 | |||
| Postop.(within 30d) ambulatory (Yes vs. No) | 0.602 | <0.001 | 0.003 2 | 2.675(1.408-5.083) | ||
| ASA (1-2 vs. 3-4) | 0.358 | 0.578 | ||||
| ESCC scale (0-1 vs. 2-3) | 0.470 | 0.268 | ||||
| Number of metastatic spine (1-2 vs. ≥3) | 0.307 | 0.795 | ||||
| Extraspinal bone metastasis (Yes vs. No) | 0.343 | 0.480 | ||||
| Visceral metastasis (Yes vs. No) | 0.280 | 0.233 | ||||
| Preop. serum albumin (<3.0 vs. ≥3.0 g/dL) | 0.072 | 0.675 | 0.857 | |||
| Intraop. bleeding (≥2000 vs. <2000 cc) | 0.377 | 0.908 | ||||
| Perioperative major complication (Yes vs. No) | 0.120 | 0.129 | 0.003 | 0.032 | 0.491 (0.256-0.940) | |
Preop: preoperative, RT: radiotherapy, CT: chemotherapy, PD: progressive disease, SD: stable disease, PR: partial remission, ECOG-PS: the Eastern Co-operative Oncology Group-Performance Status, ASA: American Society of Anesthesiologist, ESCC: epidural cord compression, HR:hazard ratio, CI: confi-dence interval, N/A: not applicable.
Table 5.
Perioperative complications analysis (within postoperative 30-day)
| Total (n=96) | P group (n=60) | W group (n=36) | P∗ | |
|---|---|---|---|---|
| Total | 23 | 15 | 8 | |
| Pneumonia | 9 | 6 | 3 | |
| Wound problems | 7 | 4 | 3 | |
| Thromboembolism | 3 | 3 | 0 | NS |
| GI bleeding | 2 | 1 | 1 | |
| Sepsis | 3 | 1 | 2 | |
| CVA | 1 | 1 | 0 | |
| Death | 10 | 7 | 3 | NS |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download