INTRODUCTION
Carotid artery stenting (CAS) is an evolving treatment method for carotid stenosis. In comparison with carotid endarterectomy (CEA), CAS can be performed without general anesthesia, involves limited surgical trauma, and does not require access to hostile neck anatomy, among other advantages. However, CAS is encumbered by a high incidence of emboli shed into the distal vascular bed. Such emboli result from plaque manipulation by guide wires, balloon inflation, and stent placement (
12).
Retinal artery occlusion (RAO) is also a potential complication after CAS, although it is not common. Only a few studies have evaluated RAO after CAS procedures, and their findings covered a broad range of incidence (4–15%) (
1). The retinal artery is a terminal artery without any anastomosis among branches and is the only source of nutrition for the inner retina. RAO and the corresponding loss of blood supply can impair visual acuity and visual fields or cause blindness in severe cases (
2). This particular complication may thus be disabling and can substantially increase socioeconomic burdens.
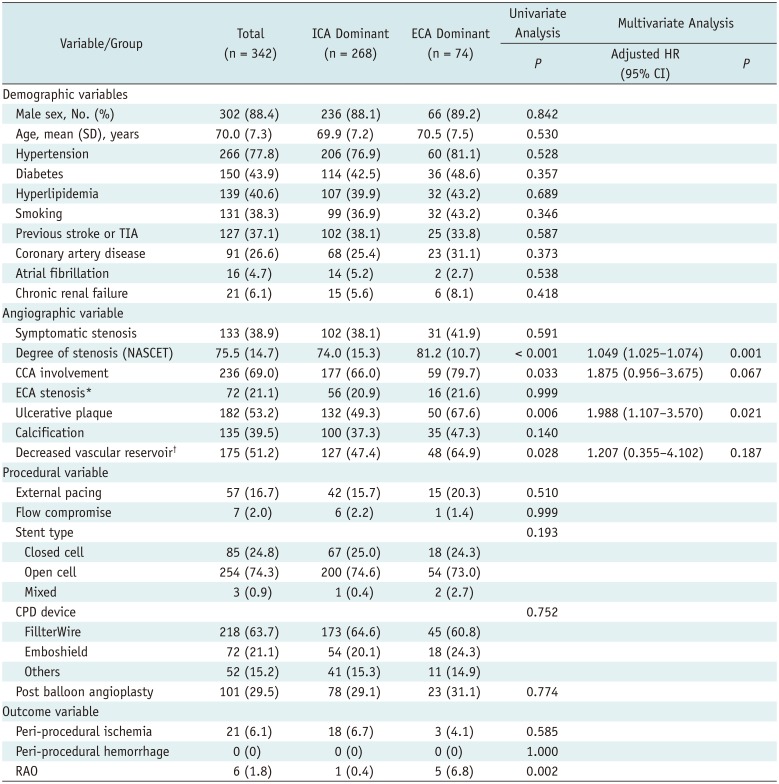
Approximately 28–35% of patients with severe carotid artery stenosis (i.e., 70–99% blockage) exhibit reversed flow to ophthalmic arteries (OAs) (
345). Reversal of OA flow calls for the development of multiple collaterals and blood supply via the external carotid artery (ECA). The OAs are then supported by ECA branches, such as the infraorbital branch of the superficial temporal artery (STA) or the orbital branch of the middle meningeal artery (MMA). During CAS procedures in patients with OAs largely supplied by the ECA (via multiple collaterals), passage of debris toward the ECA can result in the risk of RAO.
The association between RAO and OA blood supply in cases involving CAS is not well known. We anticipated that the risk of RAO was greater in patients with ECA (vs. internal carotid artery [ICA]) flow dominance. Thus, the aims of this study were to investigate the incidence of symptomatic RAO after CAS procedures and to identify the risk factors involved, focusing on the differences between ECA- and ICA-dominant OA flow.
MATERIALS AND METHODS
Study Subjects
In total, 342 consecutive CAS procedures performed at a single institution between January 2009 and December 2017 were examined in this retrospective review. All lesions were associated with atherosclerotic ICA and/or common carotid artery (CCA) stenosis. Our institutional guidelines call for CAS of stenotic ICAs in the following scenarios: severe stenosis (> 75% based on North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria) regardless of symptoms; symptomatic moderate stenosis (> 50 and ≤ 75%); or symptomatic patients with ≤ 50% lesion ulceration. CAS procedures performed for arterial dissections were excluded from this study. The primary study endpoint was RAO within 3 days after CAS. RAO was confirmed by photography and fluorescent angiography of the fundus, performed by an ophthalmologist immediately after onset of visual symptoms.
The following baseline patient parameters were examined: age, gender, hypertension, diabetes, hyperlipidemia, prior stroke or transient ischemic attack (TIA), coronary artery disease, atrial fibrillation, chronic renal failure, and smoking. The following angiographic variables were also assessed: symptomatic stenosis, degree of ipsilateral stenosis (based on NASCET criteria), CCA plaque involvement, ECA stenosis, ulcerative or calcific plaques, ipsilateral basal perfusion status on a single-photon emission computed tomography (SPECT) scan of the brain, ipsilateral vascular reservoir on a SPECT scan, and OA supply source (ECA-dominant vs. ICA-dominant group) prior to CAS. Among procedure-related variables, we analyzed external pacing, intra-procedural flow compromise, stent type (closed, open, or mixed-cell), type of cerebral protection device (CPD), and post-balloon angioplasty. In all patients treated during the study period, various types of distal filtering systems were used exclusively. Clinical outcomes were assessed in terms of peri-procedural ischemia (TIA, infarction, or RAO) and hemorrhage. This study was approved by our Institutional Review Board.
Conventional Angiography and the CAS Procedure
All stenotic lesions were detected using computed tomography angiography, magnetic resonance angiography, or carotid sonography. All patients underwent cerebral angiography and rotational angiography with three-dimensional image reconstruction via Innova IGS 630 (GE Healthcare, Chicago, IL, USA) or another system (Integris V or AlluraClarity; Philips Medical Systems, Amsterdam, The Netherlands) to assess the degree and configuration of stenosis with precision and to determine the therapeutic plans.
ECA- or ICA-dominant OA flow was confirmed by ipsilateral carotid angiography prior to CAS by assessing whether the OA was supplied chiefly and more expeditiously by the ICA or ECA. Retinochoroidal blushing from ECA collaterals (
Fig. 1) or the ICA was also assessed.
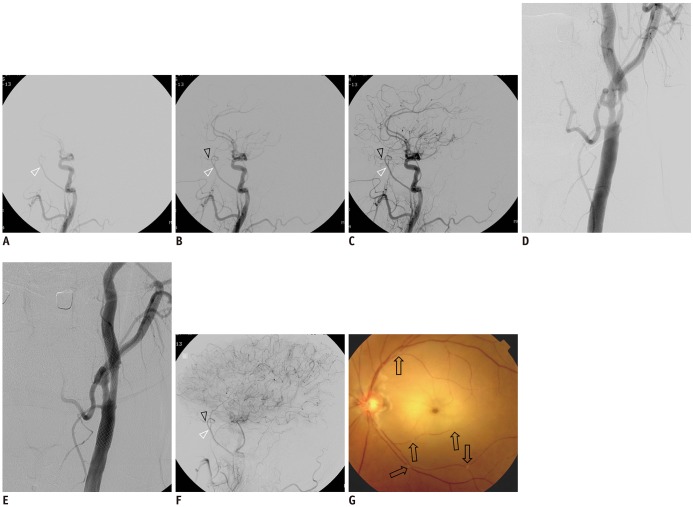
Fig. 1
Representative case with OA supplied by external carotid artery collaterals.
A–C. Sequential lateral views in common carotid angiography showing OA (black arrowheads) supplied by collateral channel via MMA (white arrowheads). D. Working projection of severely stenotic proximal internal carotid artery. E. Successful dilatation achieved after carotid angioplasty and stenting. F. After carotid artery stenting, sluggish OA (black arrowhead) flow supplied by MMA (white arrowhead). G. Fluorescent angiography of fundus showing multiple occlusions of retinal arteries (arrows). MMA = middle meningeal artery, OA = ophthalmic artery

Most CAS procedures were performed under local anesthesia. Patients were pre-treated with aspirin (100 mg/d) and clopidogrel (75 mg/d) at least 3 days before each procedure. All interventions were carried out via puncture of the femoral artery, followed by administration of heparin (70–100 U/kg) to achieve activated clotting times of 250 seconds. Long 6- or 7-Fr carotid sheaths (Flexor Shuttle-SL; Cook Medical, Bloomington, IN, USA) were advanced into the CCA and angiograms were recorded. Cerebral protection was attempted using various types of distal filtering systems at the operator's discretion and based on device availability. Once a filter was placed in the straightest possible segment, device flow status was routinely tested by contrast injection. Thereafter, pre-stent angioplasty was performed using a full-size balloon. Predilation using a 2- to 3-mm coronary balloon catheter was performed before filter placement only if angiography showed that the narrowest vessel diameter was insufficient for passage of the filter device. Self-expanding stents were delivered over the 0.014-inch filter guidewire and deployed in relatively straight arterial segments. Stent delivery and catheter retrieval after deployment were executed with caution under continuous fluoroscopic monitoring to maintain the filter's position. Various types of self-expandable stents (closed, open, or mixed-cell) were chosen according to the operator's preference or the stented arterial course. Post-deployment angiography was routinely performed to evaluate any complications or residual stenosis. If significant residual stenosis (> 30%) persisted without neurologic events or complications after stent deployment, postdilation procedures were performed using a larger balloon catheter under continuous fluoroscopic monitoring of the filter position. A retrieval catheter was then introduced over the filter wire for filter collapse and withdrawal, again using continuous fluoroscopic monitoring to avoid potential problems when crossing the stented segment. After the procedures, completion angiography was performed to confirm either distal embolic occlusion or ample perfusion. The patients continued the clopidogrel regimen for at least 90 days and the aspirin regimen indefinitely.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation. Chi-squared and Fisher's exact tests or unpaired t tests were used to assess categorical or continuous variables, respectively. To evaluate the risk factors predisposing patients to symptomatic RAO after CAS, univariate analysis was applied. Multivariate analysis was then conducted by incorporating variables with p values < 0.10 in univariate analysis. In multivariate analysis, if the sample size was small, Firth's method was used. The results of a binary logistic regression model were reported as odds ratios (ORs) with 95% confidence intervals (95% CIs) and p values. A two-tailed p value < 0.05 was considered statistically significant. All computations relied on standard software (STATA v15.0; StataCorp LP, College Station, TX, USA).
DISCUSSION
CEA is a well-established treatment for both symptomatic and asymptomatic carotid bifurcation stenosis (
6789). Increasingly, however, CAS has become a viable alternative to CEA in some instances, especially in patients with a hostile neck anatomy after surgery, tracheostomy, or irradiation or in those with anatomically high lesions (
10). The International Carotid Stenting Study, a randomized study of CAS or CEA for patients with symptomatic carotid stenosis, reported that in comparison with the CEA treatment group, approximately three times as many CAS-treated patients showed new ischemic lesions on postprocedural diffusion-weighted imaging scans (
11). Despite the usage of CPDs to lessen distal thromboembolism, debris from atherosclerotic plaques may escape CPD filters and migrate into the distal cerebral arteries during CAS procedures. Vos et al. (
1) found that emboli could be detected using transcranial Doppler sonography during wiring and passage across the stenosis, predilation, stent placement, postdilation, and use of an embolic protection device (EPD). Isolated emboli were most common during wiring and stent deployment, whereas embolic showers were primarily detected during stent deployment and postdilation.
RAO is one of potential complications of CAS. Because it is not a common complication, the mechanisms underlying post-CAS RAO are not well documented. In these cases, embolic debris may originate from both the ICA and ECA. According to Vos et al. (
1), 3 of 10 patients (30%) shielded by CPDs during treatment displayed new retinal emboli, whereas 2 of 23 patients (9%) undergoing unprotected procedures developed RAO. This suggests that CPDs placed at the ICA cannot completely filter the atherosclerotic debris loosened from plaques during CAS, or the blood flow during balloon angioplasty may flush the debris toward the retinal artery via ECA collaterals. Wilentz et al. (
12) also reported the presence of RAO after CAS using a distal ICA balloon system as CPD. As described, 5 of 38 patients (13.2%) who underwent the Theron approach (routine flushing toward the ECA) developed emboli, in comparison with 1 of 80 (1.25%) who were protected with the PercuSurge system (aspiration only; Medtronic) (
p = 0.019). Thus, embolic sources contributing to RAO are apt to migrate via ECA collaterals.
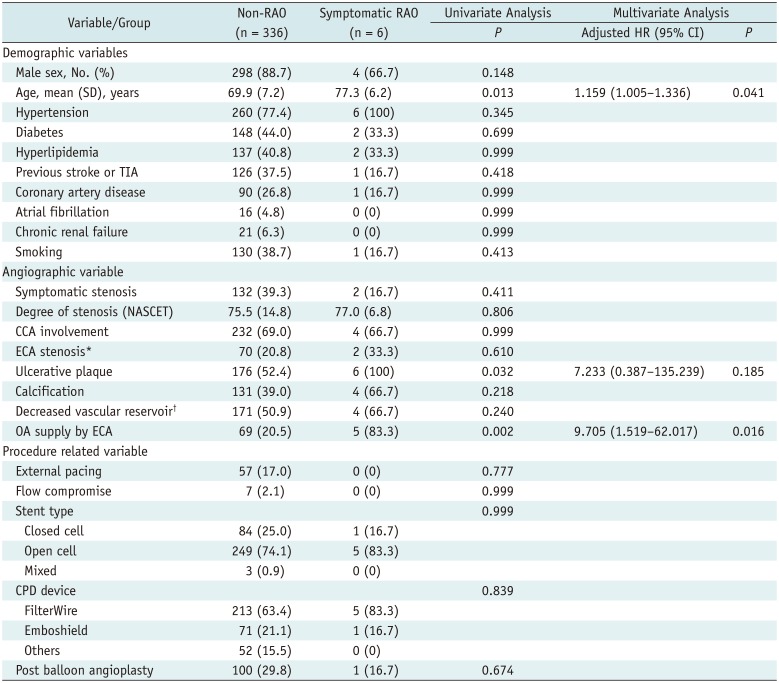
In the present study, RAO after CAS showed a significant relationship with OAs supplied by ECA collaterals prior to CAS (i.e., at baseline) and older age. In addition, OAs supplied by ECA collaterals prior to CAS were linked to severe ipsilateral ICA stenosis. Hence, OAs may be mainly supplied by the ECA in patients with severe ICA stenosis and in such patients, debris from the atherosclerotic plaque may migrate to the ECA via collaterals during balloon angioplasty (with the ICA blocked and the filter in the ICA).
According to the literature, anastomosis of the external carotid circulation to the distal ICA (e.g., orbital branch of the MMA or STA to the OA) or OA flow reversal takes place in instances of severe carotid stenosis (
34513). Debris passing through these collaterals leads to RAO and should be taken into consideration (
12). In cases of OAs supplied by the ECA, the lack of an EPD to filter debris at the ECA may also lead to RAO during CAS. The strength of our research is the premise of ECA-dominant OA flow. Indeed, we have now demonstrated a significant relationship between ECA-dominant OA flow and symptomatic RAO. Thus, to avoid RAO as a procedural complication, it is vital to check whether the OA is largely supplied by the ICA or ECA prior to CAS.
Given the above revelations, any currently popular distal filtering system that is placed in the ICA cannot completely prevent RAO after CAS. The Mo.Ma model (Medtronic) is one type of proximal CPD that works by blocking flow in a target vessel through balloon occlusion of the ECA and CCA. In patients with severe stenosis plus ECA-dominant OA supply, the Mo.MA CPD may prevent RAO after CAS by averting the rush of debris into ECA collaterals and permitting suction removal. One drawback of this device is occlusion intolerance, which occurs at a rate of 12% (
14). However, if collateral circulation through the Circle of Willis is sufficient, the Mo.Ma CPD may be a good option for preventing RAO in this particular context.
The incidence of RAO after CAS varies across reports, of which there are few. Song et al. (
2) reported a 4.9% rate of RAO in their CEA group (n = 61), unlike the much higher 16.9% rate in their CAS group (n = 71) (
p = 0.031). In the aftermath of CAS, 12 patients exhibited RAO, but only a single patient experienced diminished visual acuity or visual field (incidence of symptomatic RAO, 1.4%). According to Vos et al. (
1), RAO occurred after CAS in 5 of 33 procedures (15%), all of which proved asymptomatic. Fortunately, most retinal emboli are clinically silent (
15). Wilentz et al. (
12) have also reported a 5.1% (6/116) incidence of RAO after CAS, whereas the incidence of symptomatic RAO was just 1.7% (2/116). In our series, the incidence of symptomatic RAO following CAS was 1.8%, which is consistent with the outcomes of other studies.
Certain limitations of this study need to be acknowledged. First, this was a retrospective observational study with few occurrences of symptomatic RAO, despite the large sample size. In addition, the stents and distal filter systems used as CPDs were not standardized. Furthermore, the ophthalmologic examinations stipulated were not routinely performed in all patients with CAS. Only those experiencing visual symptoms after CAS underwent the required battery of studies.
In conclusion, the incidence of symptomatic RAO after CAS was low (1.8%) in our population. However, because reversal of OA flow to the ECA (established prior to stenting of severely stenotic ICAs) proved independently predictive of symptomatic RAO after CAS, caution should be exercised in this setting. For older patients, the use of simultaneous ICA-ECA CPDs may help prevent such complications.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download