1. National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409. PMID:
21714641.

2. Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009; 253:606–622. PMID:
19952025.

3. Infante M, Lutman RF, Imparato S, Di Rocco M, Ceresoli GL, Torri V, et al. Differential diagnosis and management of focal ground-glass opacities. Eur Respir J. 2009; 33:821–827. PMID:
19047318.

4. Mack MJ, Aronoff RJ, Acuff TE, Douthit MB, Bowman RT, Ryan WH. Present role of thoracoscopy in the diagnosis and treatment of diseases of the chest. Ann Thorac Surg. 1992; 54:403–408. discussion 407–409. PMID:
1510505.

5. Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999; 115:563–568. PMID:
10027460.
6. Plunkett MB, Peterson MS, Landreneau RJ, Ferson PF, Posner MC. Peripheral pulmonary nodules: preoperative percutaneous needle localization with CT guidance. Radiology. 1992; 185:274–276. PMID:
1523323.

7. Finley RJ, Mayo JR, Grant K, Clifton JC, English J, Leo J, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg. 2015; 149:26–31. PMID:
25293355.

8. Park CH, Han K, Hur J, Lee SM, Lee JW, Hwang SH, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. 2017; 151:316–328. PMID:
27717643.
9. Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis. 2016; 8(Suppl 9):S749–S755. PMID:
28066679.

10. Zaman M, Bilal H, Woo CY, Tang A. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg. 2012; 15:266–272. PMID:
22572410.

11. Horan TA, Pinheiro PM, Araújo LM, Santiago FF, Rodrigues MR. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg. 2002; 73:1647–1649. PMID:
12022575.

12. Sakiyama S, Kondo K, Matsuoka H, Yoshida M, Miyoshi T, Yoshida S, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg. 2003; 126:1207–1209. PMID:
14566279.

13. Asamura H, Kondo H, Naruke T, Tsuchiya R, Wakao F, Kaneko M, et al. Computed tomography-guided coil injection and thoracoscopic pulmonary resection under roentgenographic fluoroscopy. Ann Thorac Surg. 1994; 58:1542–1544. PMID:
7979696.

14. Su TH, Fan YF, Jin L, He W, Hu LB. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol. 2015; 25:2627–2633. PMID:
25773939.

15. Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology. 2009; 250:576–585. PMID:
19188326.

16. Sui X, Zhao H, Yang F, Li JL, Wang J. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis. 2015; 7:1580–1587. PMID:
26543605.
17. Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol. 1994; 163:297–300. PMID:
7518642.

18. Keating J, Singhal S. Novel methods of intraoperative localization and margin assessment of pulmonary nodules. Semin Thorac Cardiovasc Surg. 2016; 28:127–136. PMID:
27568150.

19. Vandoni RE, Cuttat JF, Wicky S, Suter M. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998; 14:265–270. PMID:
9761435.

20. Lee NK, Park CM, Kang CH, Jeon YK, Choo JY, Lee HJ, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol. 2012; 13:694–701. PMID:
23118567.

21. Okumura T, Kondo H, Suzuki K, Asamura H, Kobayashi T, Kaneko M, et al. Fluoroscopy-assisted thoracoscopic surgery after computed tomography-guided bronchoscopic barium marking. Ann Thorac Surg. 2001; 71:439–442. PMID:
11235684.

22. Asano F, Shindoh J, Shigemitsu K, Miya K, Abe T, Horiba M, et al. Ultrathin bronchoscopic barium marking with virtual bronchoscopic navigation for fluoroscopy-assisted thoracoscopic surgery. Chest. 2004; 126:1687–1693. PMID:
15539745.

23. Kobayashi T, Kaneko M, Kondo H, Nakayama H, Asamura H, Tsuchiya R, et al. CT-guided bronchoscopic barium marking for resection of a fluoroscopically invisible peripheral pulmonary lesion. Jpn J Clin Oncol. 1997; 27:204–205. PMID:
9255280.

24. Nomori H, Horio H, Naruke T, Suemasu K. Fluoroscopy-assisted thoracoscopic resection of lung nodules marked with lipiodol. Ann Thorac Surg. 2002; 74:170–173. PMID:
12118752.

25. Moon SW, Wang YP, Jo KH, Kwack MS, Kim SW, Kwon OK, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg. 1999; 68:1815–1820. PMID:
10585064.

26. Kawanaka K, Nomori H, Mori T, Ikeda K, Ikeda O, Tomiguchi S, et al. Marking of small pulmonary nodules before thoracoscopic resection: injection of lipiodol under CT-fluoroscopic guidance. Acad Radiol. 2009; 16:39–45. PMID:
19064210.
27. Kim YD, Jeong YJ, I H, Cho JS, Lee JW, Kim HJ, et al. Localization of pulmonary nodules with lipiodol prior to thoracoscopic surgery. Acta Radiol. 2011; 52:64–69. PMID:
21498328.

28. Ambrogi MC, Melfi F, Zirafa C, Lucchi M, De Liperi A, Mariani G, et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc. 2012; 26:914–919. PMID:
22011947.

29. Chella A, Lucchi M, Ambrogi MC, Menconi G, Melfi FM, Gonfiotti A, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg. 2000; 18:17–21. PMID:
10869935.

30. Starnes SL, Wolujewicz M, Guitron J, Williams V, Scheler J, Ristagno R. Radiotracer localization of nonpalpable pulmonary nodules: a single-center experience. J Thorac Cardiovasc Surg. 2018; 156:1986–1992. PMID:
29778333.

31. Bellomi M, Veronesi G, Trifirò G, Brambilla S, Bonello L, Preda L, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg. 2010; 90:1759–1764. PMID:
21095303.

32. Koike T, Koike T, Yoshiya K, Tsuchida M, Toyabe S. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013; 146:372–378. PMID:
23870323.

33. El-Sherif A, Fernando HC, Santos R, Pettiford B, Luketich JD, Close JM, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007; 14:2400–2405. PMID:
17505859.

34. Wolf AS, Swanson SJ, Yip R, Liu B, Tarras ES, Yankelevitz DF, et al. I-ELCAP Investigators. The impact of margins on outcomes after wedge resection for stage I non-small cell lung cancer. Ann Thorac Surg. 2017; 104:1171–1178. PMID:
28669499.

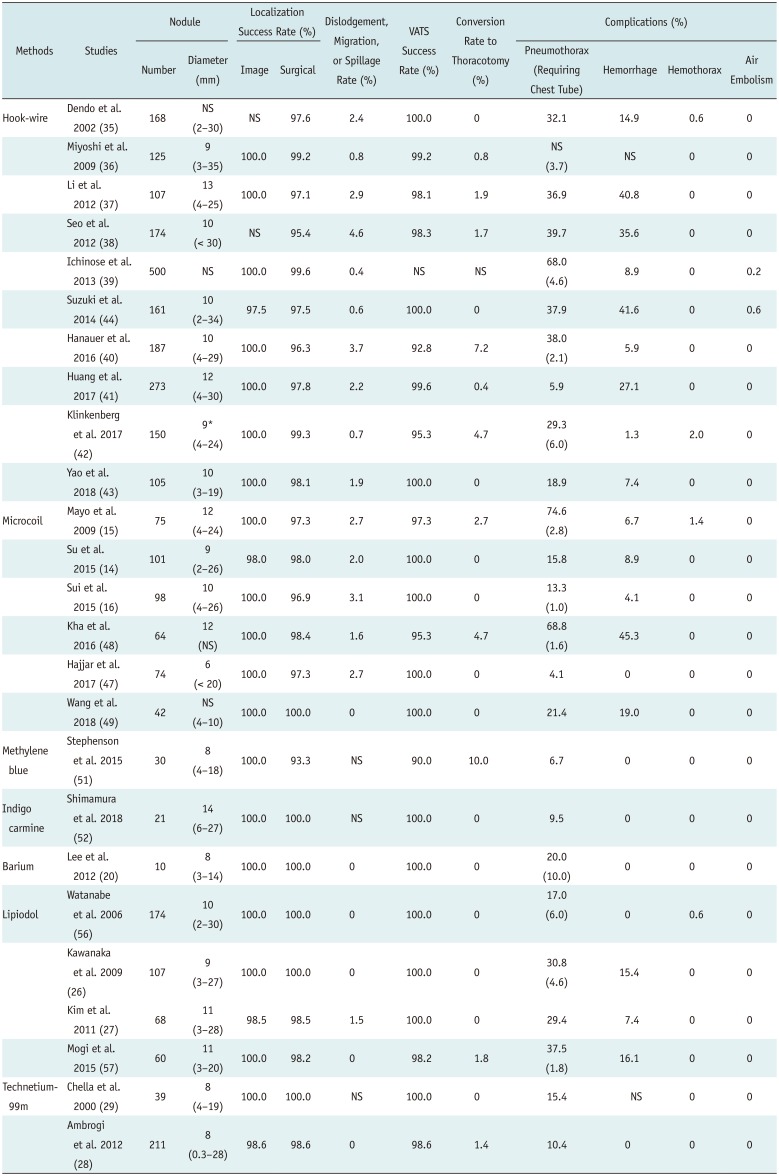
35. Dendo S, Kanazawa S, Ando A, Hyodo T, Kouno Y, Yasui K, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology. 2002; 225:511–518. PMID:
12409589.

36. Miyoshi K, Toyooka S, Gobara H, Oto T, Mimura H, Sano Y, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg. 2009; 36:378–382. PMID:
19414272.

37. Li W, Wang Y, He X, Li G, Wang S, Xu L, et al. Combination of CT-guided hookwire localization and video-assisted thoracoscopic surgery for pulmonary nodular lesions: analysis of 103 patients. Oncol Lett. 2012; 4:824–828. PMID:
23205107.

38. Seo JM, Lee HY, Kim HK, Choi YS, Kim J, Shim YM, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg. 2012; 143:809–814. PMID:
22104686.

39. Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg. 2013; 96:1203–1208. PMID:
23895891.

40. Hanauer M, Perentes JY, Krueger T, Ris HB, Bize P, Schmidt S, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg. 2016; 11:5. PMID:
26772183.

41. Huang HZ, Wang GZ, Xu LC, Li GD, Wang Y, Wang YH, et al. CT-guided Hookwire localization before video-assisted thoracoscopic surgery for solitary ground-glass opacity dominant pulmonary nodules: radiologic-pathologic analysis. Oncotarget. 2017; 8:108118–108129. PMID:
29296228.

42. Klinkenberg TJ, Dinjens L, Wolf RFE, van der Wauwer C, van der Wekken AJ, de Bock GH, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol. 2017; 115:898–904. PMID:
28230245.

43. Yao F, Wang J, Yao J, Xu L, Wang J, Gao L. Reevaluation of the efficacy of preoperative computed tomography-guided hook wire localization: a retrospective analysis. Int J Surg. 2018; 51:24–30. PMID:
29367030.

44. Suzuki K, Shimohira M, Hashizume T, Ozawa Y, Sobue R, Mimura M, et al. Usefulness of CT-guided hookwire marking before video-assisted thoracoscopic surgery for small pulmonary lesions. J Med Imaging Radiat Oncol. 2014; 58:657–662. PMID:
25088355.

45. Iguchi T, Hiraki T, Gobara H, Fujiwara H, Matsui Y, Sugimoto S, et al. Simultaneous multiple preoperative localizations of small pulmonary lesions using a short hook wire and suture system. Cardiovasc Intervent Radiol. 2015; 38:971–976. PMID:
25465062.

46. Mullan BF, Stanford W, Barnhart W, Galvin JR. Lung nodules: improved wire for CT-guided localization. Radiology. 1999; 211:561–565. PMID:
10228543.

47. Hajjar W, Al-Nassar S, Almousa O, Rahal S, Al-Aqeed A, Ahmed I, et al. Thoracoscopic resection of suspected metastatic pulmonary nodules after microcoil localization technique: a prospective study. J Cardiovasc Surg (Torino). 2017; 58:606–612.

48. Kha LC, Hanneman K, Donahoe L, Chung T, Pierre AF, Yasufuku K, et al. Safety and efficacy of modified preoperative lung nodule microcoil localization without pleural marking: a pilot study. J Thorac Imaging. 2016; 31:15–22. PMID:
26502347.
49. Wang ZX, Li L, Zhang Z, Wang GH, Kong DM, Wang XD, et al. High-resolution computed tomography features and CT-guided microcoil localization of subcentimeter pulmonary ground-glass opacities: radiological processing prior to video-assisted thoracoscopic surgery. J Thorac Dis. 2018; 10:2676–2684. PMID:
29997929.

50. Sui X, Zhao H, Yang F, Liu G, Hu L, Chen C, et al. Analysis of factors affecting successful microcoil localization for pulmonary nodules. J Surg Res. 2018; 224:193–199. PMID:
29506840.

51. Stephenson JA, Mahfouz A, Rathinam S, Nakas A, Bajaj A. A simple and safe technique for CT guided lung nodule marking prior to video assisted thoracoscopic surgical resection revisited. Lung Cancer Int. 2015; 2015:235720. PMID:
26579236.

52. Shimamura Y, Sasaki S, Shimohira M, Ogino H, Yuki D, Nakamae K, et al. New technique of percutaneous CT fluoroscopy-guided marking before video-assisted thoracoscopic surgery for small lung lesions: feasibility of using a 25-gauge needle without local anaesthesia. Br J Radiol. 2018; 91:20170692. PMID:
29172683.

53. Lin MW, Tseng YH, Lee YF, Hsieh MS, Ko WC, Chen JY, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg. 2016; 152:535–544.e2. PMID:
27189890.

54. McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg. 2002; 37:1729–1731. PMID:
12483642.

55. Kleedehn M, Kim DH, Lee FT, Lubner MG, Robbins JB, Ziemlewicz TJ, et al. Preoperative pulmonary nodule localization: a comparison of methylene blue and hookwire techniques. AJR Am J Roentgenol. 2016; 207:1334–1339. PMID:
27657546.

56. Watanabe K, Nomori H, Ohtsuka T, Kaji M, Naruke T, Suemasu K. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg. 2006; 132:320–324. PMID:
16872957.

57. Mogi A, Yajima T, Tomizawa K, Onozato R, Tanaka S, Kuwano H. Video-assisted thoracoscopic surgery after preoperative CT-guided lipiodol marking of small or impalpable pulmonary nodules. Ann Thorac Cardiovasc Surg. 2015; 21:435–439. PMID:
26004116.

58. Miura H, Yamagami T, Tanaka O, Yoshimatsu R, Ichijo Y, Kato D, et al. CT findings after lipiodol marking performed before video-assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol. 2016; 57:303–310. PMID:
25795703.

59. Doo KW, Yong HS, Kim HK, Kim S, Kang EY, Choi YH. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol. 2015; 22:331–337. PMID:
25008029.

60. Iguchi T, Hiraki T, Gobara H, Fujiwara H, Matsui Y, Miyoshi S, et al. CT fluoroscopy-guided preoperative short hook wire placement for small pulmonary lesions: evaluation of safety and identification of risk factors for pneumothorax. Eur Radiol. 2016; 26:114–121. PMID:
25991483.

61. Nour-Eldin NE, Alsubhi M, Naguib NN, Lehnert T, Emam A, Beeres M, et al. Risk factor analysis of pulmonary hemorrhage complicating CT-guided lung biopsy in coaxial and non-coaxial core biopsy techniques in 650 patients. Eur J Radiol. 2014; 83:1945–1952. PMID:
25063212.

62. Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011; 196:W678–W682. PMID:
21606253.

63. Cham MD, Lane ME, Henschke CI, Yankelevitz DF. Lung biopsy: special techniques. Semin Respir Crit Care Med. 2008; 29:335–349. PMID:
18651353.

64. Yi JH, Choi PJ, Bang JH, Jeong SS, Cho JH. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis. 2018; 10:E59–E64. PMID:
29600106.

65. Endo M, Kotani Y, Satouchi M, Takada Y, Sakamoto T, Tsubota N, et al. CT fluoroscopy-guided bronchoscopic dye marking for resection of small peripheral pulmonary nodules. Chest. 2004; 125:1747–1752. PMID:
15136386.

66. Krimsky WS, Minnich DJ, Cattaneo SM, Sarkar SA, Harley DP, Finley DJ, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect. 2014; 2. 17. [Epub]. DOI:
10.3402/jchimp.v4.23084.

67. Tay JH, Wallbridge PD, Larobina M, Russell PA, Irving LB, Steinfort DP. Electromagnetic navigation bronchoscopydirected pleural tattoo to aid surgical resection of peripheral pulmonary lesions. J Bronchology Interv Pulmonol. 2016; 23:245–250. PMID:
26496089.

68. Kuo SW, Tseng YF, Dai KY, Chang YC, Chen KC, Lee JM. Electromagnetic navigation bronchoscopy localization versus percutaneous CT-guided localization for lung resection via video-assisted thoracoscopic surgery: a propensity-matched study. J Clin Med. 2019; 8:E379. PMID:
30889927.

69. Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg. 2014; 98:471–475. discussion 475–476. PMID:
24968769.

70. Marino KA, Sullivan JL, Weksler B. Electromagnetic navigation bronchoscopy for identifying lung nodules for thoracoscopic resection. Ann Thorac Surg. 2016; 102:454–457. PMID:
27173068.

71. Georgiou HD, Taverner J, Irving LB, Steinfort DP. Safety and efficacy of radial EBUS for the investigation of peripheral pulmonary lesions in patients with advanced COPD. J Bronchology Interv Pulmonol. 2016; 23:192–198. PMID:
27454473.

72. Wada H, Anayama T, Hirohashi K, Nakajima T, Kato T, Waddell TK, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur J Cardiothorac Surg. 2016; 49:690–697. PMID:
25855597.

73. Kondo R, Yoshida K, Hamanaka K, Hashizume M, Ushiyama T, Hyogotani A, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg. 2009; 138:837–842. PMID:
19660350.

74. Piolanti M, Coppola F, Papa S, Pilotti V, Mattioli S, Gavelli G. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol. 2003; 13:2358–2364. PMID:
12736756.

75. Mattioli S, D'Ovidio F, Daddi N, Ferruzzi L, Pilotti V, Ruffato A, et al. Transthoracic endosonography for the intraoperative localization of lung nodules. Ann Thorac Surg. 2005; 79:443–449. discussion 443–449. PMID:
15680811.

76. Khereba M, Ferraro P, Duranceau A, Martin J, Goudie E, Thiffault V, et al. Thoracoscopic localization of intraparenchymal pulmonary nodules using direct intracavitary thoracoscopic ultrasonography prevents conversion of VATS procedures to thoracotomy in selected patients. J Thorac Cardiovasc Surg. 2012; 144:1160–1165. PMID:
22980667.

77. Sortini D, Feo CV, Carcoforo P, Carrella G, Pozza E, Liboni A, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule and history of malignancy. Ann Thorac Surg. 2005; 79:258–262. discussion 262. PMID:
15620953.

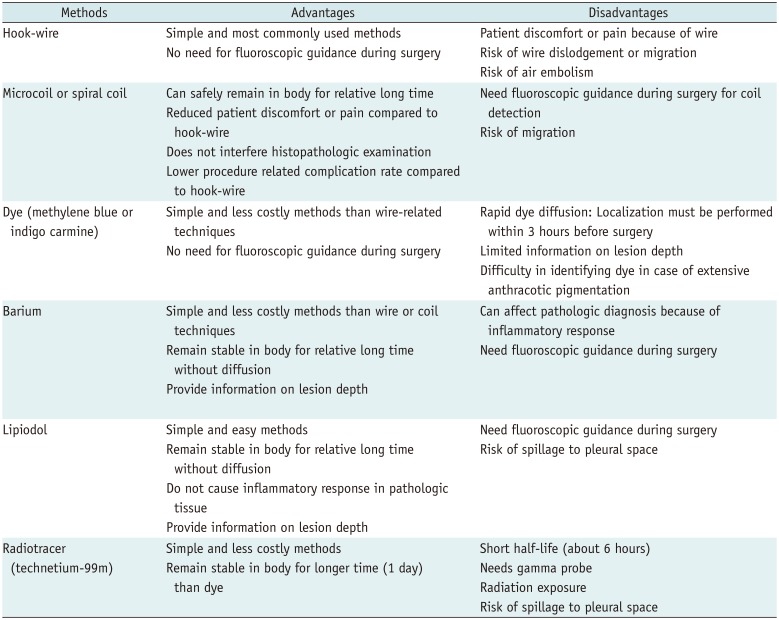
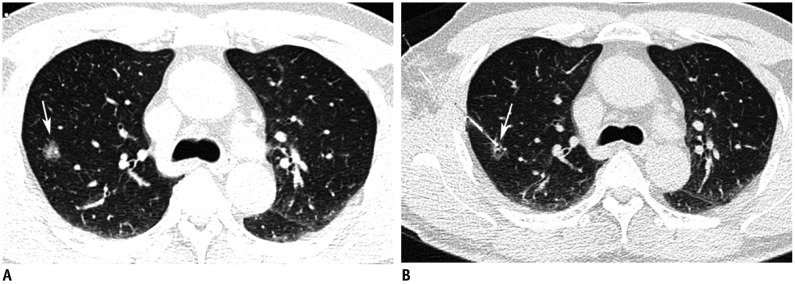
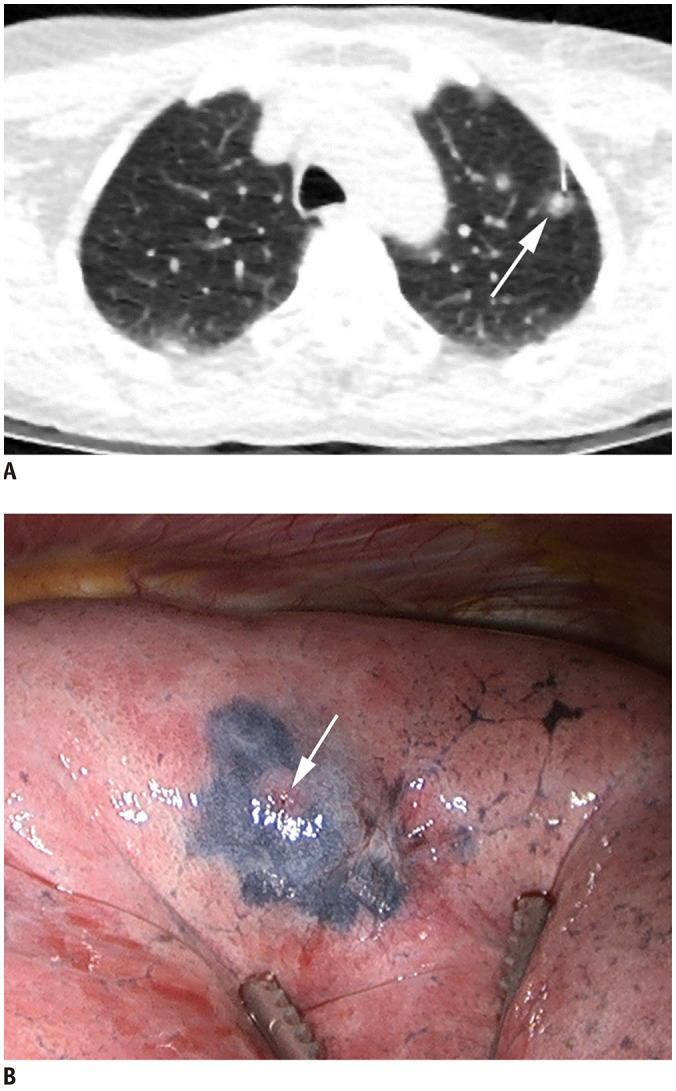
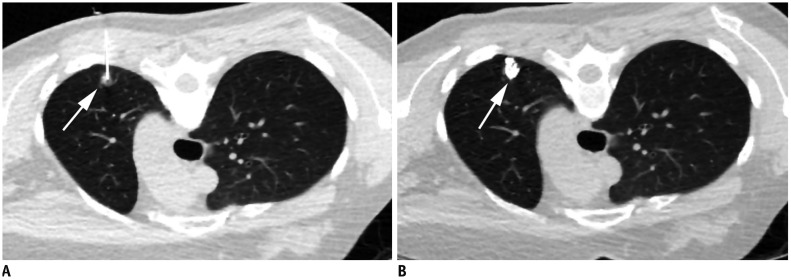




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download