This article has been
cited by other articles in ScienceCentral.
Dear Editor,
A 37-year-old male patient was admitted to the emergency room 3 days after last being seen well. He had been found in his apartment at a reduced level of consciousness, with foam in his mouth and enuresis. His tympanic temperature was 40℃. The patient was intubated immediately for airway protection. Brain CT showed mild brain atrophy, a focal subarachnoid hemorrhage in the left frontal lobe, and scattered hypodensities in the frontal and parietal white matter bilaterally. A CT scan of the chest showed pulmonary infiltrates of the left lung. Toxicology was positive only for cannabinoids.
A CSF analysis revealed a cell count of 21,980/µL (90% neutrophils), protein >2,000 mg/L, glucose <1 mg/dL, and lactate=14.38 mmol/L, which was consistent with bacterial meningitis. Streptococcus pneumoniae was identified in blood cultures. The patient was treated with intravenous penicillin G in combination with clarithromycin and dexamethasone. An external ventricular drain was placed to monitor the intracranial pressure. Negative findings were obtained in further diagnostic investigations for detecting a parameningeal focus or an immunocompromised condition, including CT scans of the paranasal sinus and mastoid cells, whole-body CT scan, and transesophageal echocardiography as well as HIV/hepatitis B/hepatitis C/syphilis serology.
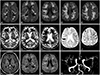
Analgosedation was stopped on day 1. The neurological examination performed on day 3 revealed that the patient remained comatose, intubated, and breathing spontaneously with pressure support, had preserved brain stem reflexes, and responded with extension posturing to pain. Cranial MRI was performed on day 5. Diffusion-weighted images (DWI) demonstrated pyogenic ventriculitis and extensive subcortical areas of restriction in DWI and decreased apparent diffusion coefficient (
Fig. 1) with corresponding hyperintense signal on FLAIR images. Contrast-enhanced T1-weighted images demonstrated slight leptomeningeal enhancement without disruption of the parenchymal blood-brain barrier. Vasculitis was indicated by focal narrowing of several intracranial arteries.
The patient did not exhibit any seizures, and prophylactic anticonvulsive therapy was not started. On day 10 he showed a syndrome of unresponsive wakefulness despite receiving adequate antibiotic treatment, with a CSF cell count of 10/µL. The prognosis was judged to be highly unfavorable, and life-supporting measures were withdrawn on day 11. The patient died 2 days later.
This patient represents the first reported fatal case of pneumococcal meningoencephalitis with widespread symmetrical white-matter lesions. Intracranial complications in pneumococcal meningitis are frequent and contribute to the high mortality rate of about 20%, with cerebrovascular complications due to necrotizing vasculitis previously observed in 21.8% of 87 patients.
1 MRI angiography performed in our patient demonstrated focal narrowing of the intracranial midsize arteries, which was consistent with vasculitis. The detection of focal areas of subarachnoid hemorrhage as well as ischemic lesions in the course of lenticulostriate arteries along the external and internal capsule add to the clinical picture. Symmetrical ischemic lesions due to vasculitis involving the internal capsule were also observed by Vernino et al.
2 However, the extensive white-matter lesions with a confluent lesion pattern and sparing of the cortex observed in our patient greatly exceed focal ischemia caused by vasculitis. Few case reports have referred to widespread white-matter lesions in pneumococcal meningitis, instead being limited either to confluent lesions of the deep white matter
3 or to symmetrical lesions predominantly of the frontal white matter.
4 As a pathophysiological correlate of these areas, Jorens et al.
3 reported on small-vessel disease and cytotoxic edema due to global ischemia. We believe that the extended white-matter lesions in our patient corresponded to cytotoxic edema attributable to parainfectious inflammatory syndromes, and accounted for the lack of clinical improvement despite adequate antibiotic treatment being administered. In addition to the aforementioned ischemic lesions due to vasculitis in the course of lenticulostriate arteries, the larger extent of neuronal damage with resulting cytotoxic edema may be ascribed to injury due to the release of proinflammatory and toxic compounds, the stimulation of the innate immune system, and glial stimulation and toxicity.
5 Parainfectious acute disseminated encephalomyelitis (ADEM) has also been described in the context of pneumococcal meningitis, and typically presents with widespread T2-weighted hyperintense lesions in the subcortical and central white matter.
6 However, lesions in ADEM are mostly poorly marginated and asymmetrical.
7 Moreover, vasogenic edema is a hallmark of (pediatric) ADEM, whereas cytotoxic edema is rare.
8 Nevertheless, we cannot exclude that the inflammatory lesions in our case and postpneumococcal ADEM are different manifestations of the same spectrum of diseases.
In summary, performing MRI may facilitate the outcome prognostication in patients with pneumococcal meningitis and persistent severe neurological impairment.
The study was approved by the local Institutional Review Board (Medizinische Ethikkommission II der Medizinischen Fakultät Mannheim, University of Heidelberg) and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patient consent was not required due to its retrospective design and the lack of patient interaction.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download