1. Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005; 12:747–758. PMID:
16190912.

2. Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst. 2010; 15:79–92. PMID:
20626771.
3. Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912–1925. PMID:
18524793.

4. Mellgren SI, Nolano M, Sommer C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol. 2013; 115:171–188. PMID:
23931780.
5. Lauria G. Small fiber neuropathies. Curr Opin Neurol. 2005; 18:591–597. PMID:
16155446.
6. Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013; 497:332–337. PMID:
23575631.

7. Lee E, Choi J, Jo Y, Kim JY, Jang YJ, Lee HM, et al. ACT-PRESTO: rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci Rep. 2016; 6:18631. PMID:
26750588.

8. Gong H, Zeng S, Yan C, Lv X, Yang Z, Xu T, et al. Continuously tracing brain-wide long-distance axonal projections in mice at a one-micron voxel resolution. Neuroimage. 2013; 74:87–98. PMID:
23416252.

9. Li A, Gong H, Zhang B, Wang Q, Yan C, Wu J, et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain. Science. 2010; 330:1404–1408. PMID:
21051596.

10. Kennedy WR, Wendelschafer-Crabb G. The innervation of human epidermis. J Neurol Sci. 1993; 115:184–190. PMID:
8482979.

11. McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995; 45:1848–1855. PMID:
7477980.

12. Gammon WR, Briggaman RA, Inman AO 3rd, Queen LL, Wheeler CE. Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol. 1984; 82:139–144. PMID:
6363567.
13. Rowbotham MC, Yosipovitch G, Connolly MK, Finlay D, Forde G, Fields HL. Cutaneous innervation density in the allodynic form of postherpetic neuralgia. Neurobiol Dis. 1996; 3:205–214. PMID:
8980021.

14. Oaklander AL, Romans K, Horasek S, Stocks A, Hauer P, Meyer RA. Unilateral postherpetic neuralgia is associated with bilateral sensory neuron damage. Ann Neurol. 1998; 44:789–795. PMID:
9818935.

15. Boucek P, Havrdova T, Voska L, Lodererova A, He L, Saudek F, et al. Epidermal innervation in type 1 diabetic patients: a 2.5-year prospective study after simultaneous pancreas/kidney transplantation. Diabetes Care. 2008; 31:1611–1612. PMID:
18443196.
16. Schifitto G, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, et al. Long-term treatment with recombinant nerve growth factor for HIV-associated sensory neuropathy. Neurology. 2001; 57:1313–1316. PMID:
11591856.

17. Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005; 12:932–940.

18. Tang J, Germain RN, Cui M. Superpenetration optical microscopy by iterative multiphoton adaptive compensation technique. Proc Natl Acad Sci U S A. 2012; 109:8434–8439. PMID:
22586078.

19. Tomer R, Ye L, Hsueh B, Deisseroth K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc. 2014; 9:1682–1697. PMID:
24945384.

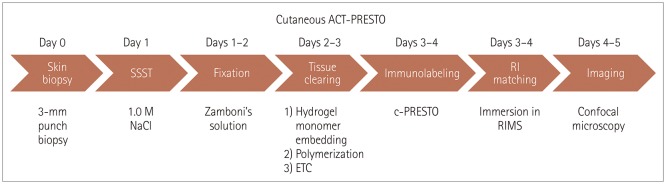
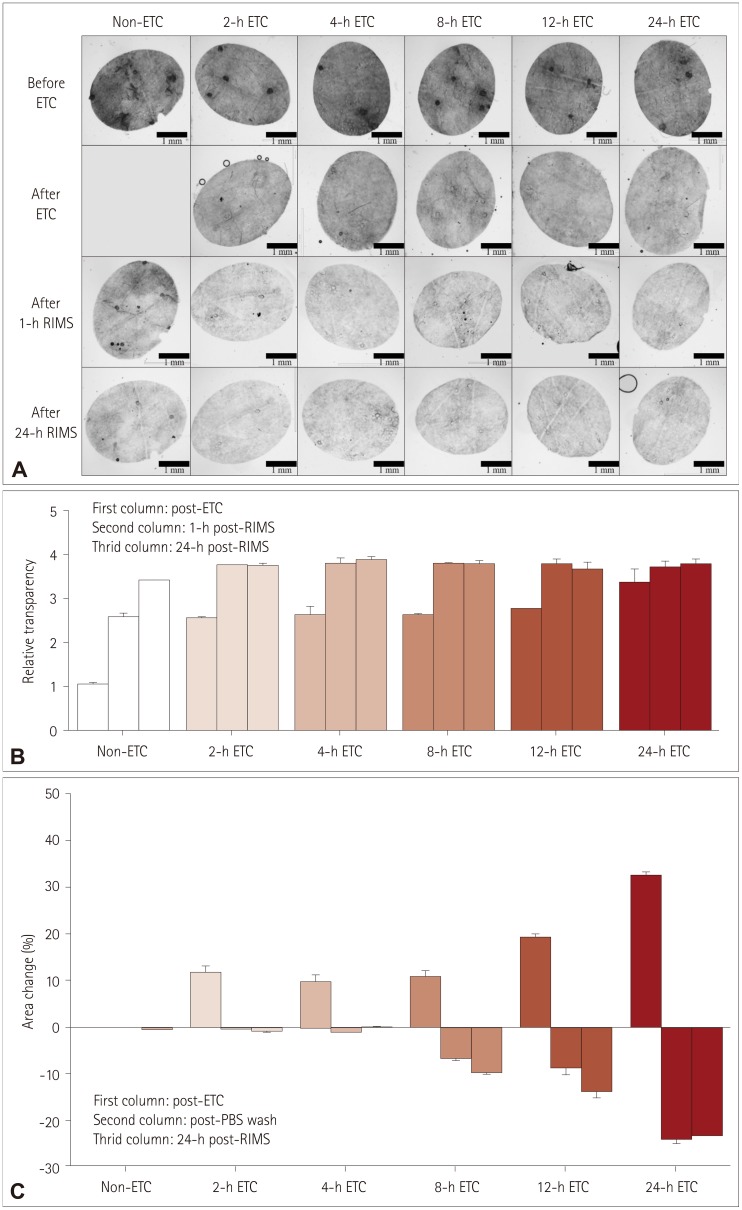
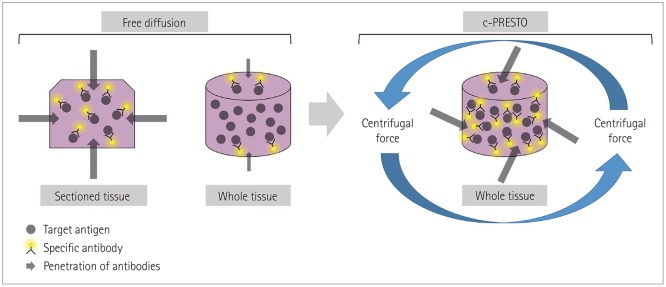
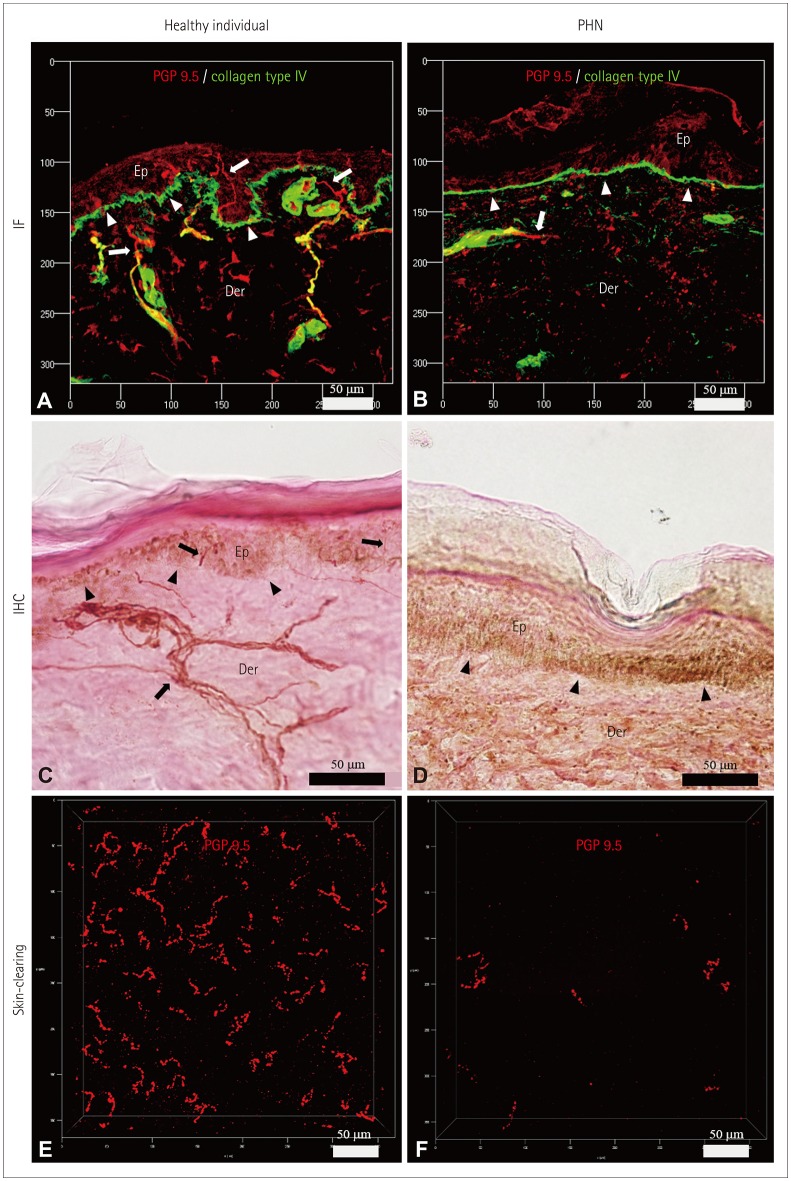




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download