Abstract
Objectives
The purpose of this study was to investigate changes in the composition of artificial cariogenic biofilms using a Streptococcus mutans biofilm model over a period of time.
Methods
We analyzed the dry weight, colony forming unit (CFU) number, extracellular polysaccharide (EPS) biovolume, and acid production rate of S. mutans biofilms formed on saliva-coated hydroxyapatite discs after 26 h, 50 h, 74 h, 98 h, 171 h, and 195 h. In addition, we performed a laser scanning confocal fluorescence microscopy to determine the bacterial volume, EPS biovolume, and biofilm thickness. We calculated the biofilm density using dry weight and EPS biovolume.
Results
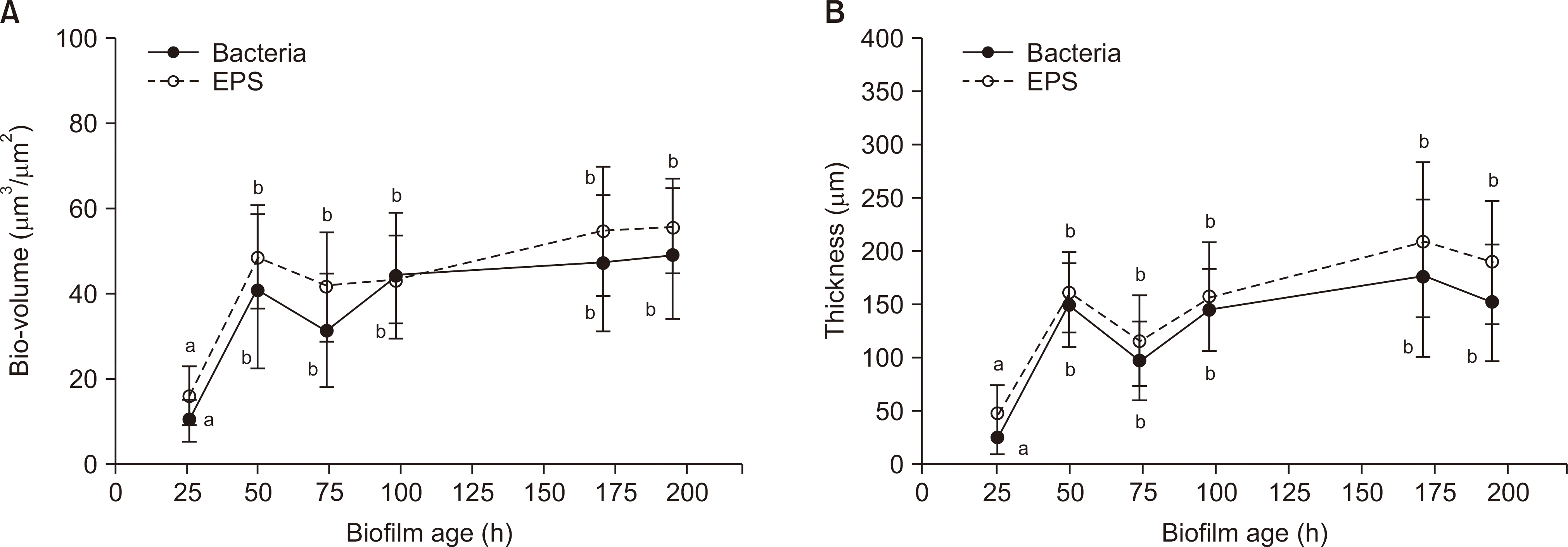
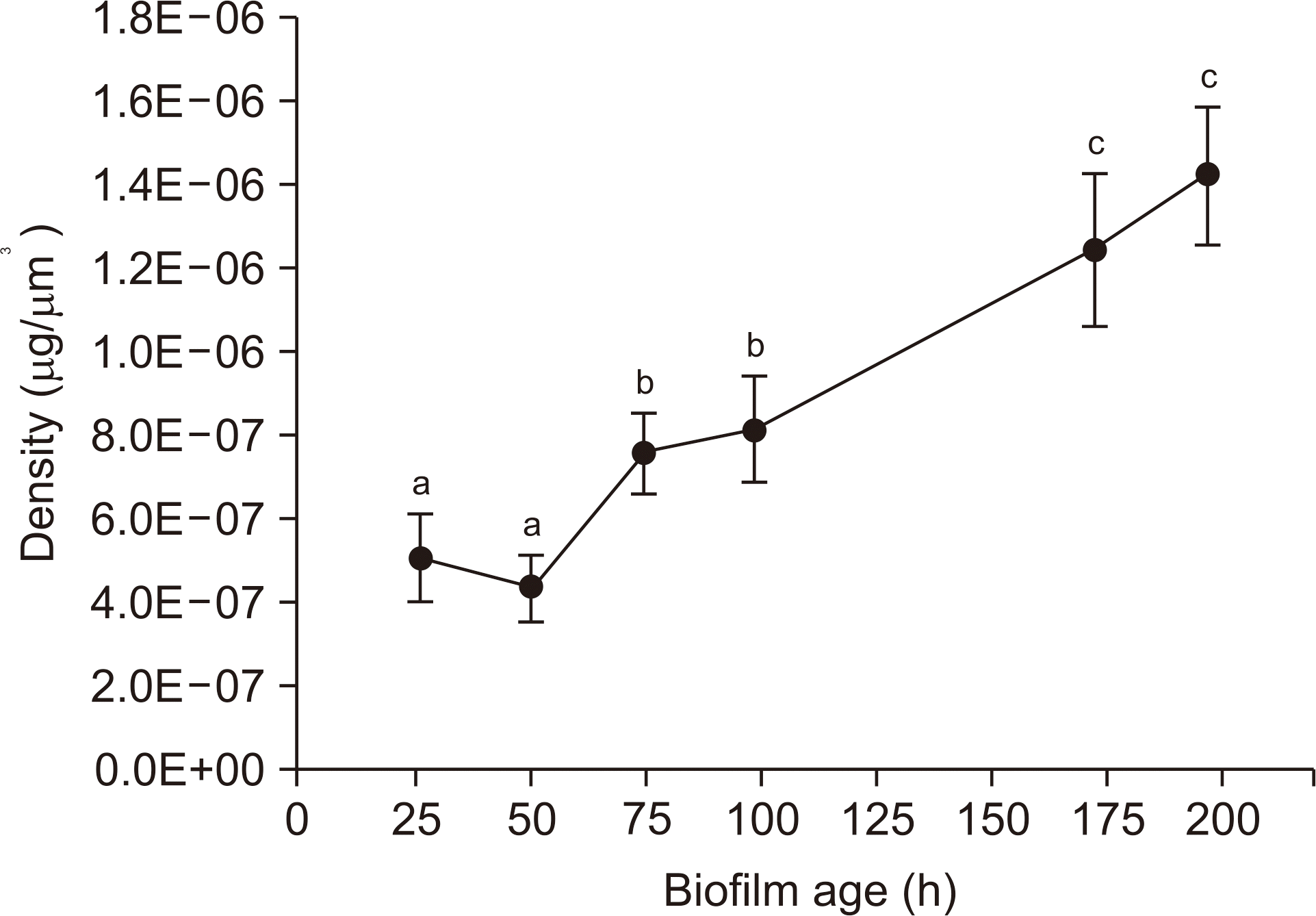
Over a period of time, there was no change in the CFU number and acid production rate of S. mutans biofilms, but there was an increase in the dry weight and EPS biovolume of S. mutans biofilms. The bacterial volume, EPS biovolume, and biofilm thickness only increased in the 50-hold biofilm; however, no change was observed in 50-195-h-old biofilms. In addition, an increase in the biofilm density was observed over time.
Conclusions
These results suggest that the acid production ability of cariogenic biofilms does not change, but the biofilm density increases over time. However, due to scientific information, further research needs to be conducted in the field of dentistry to get further insights on the progression of cariogenic biofilms over time.
Go to : 
References
1. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003; 149:279–294.

2. Jeon JG, Rosalen PL, Falsetta ML, Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 2011; 45:243–263.

3. Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011; 45:69–86.
4. Palmer RJ. Composition and development of oral bacterial communities. Periodontol 2000. 2014; 64:20–39.
5. Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011; 55:16–35.

6. Cook LC, Dunny GM. The influence of biofilms in the biology of plasmids. Microbiol Spectr. 2014; 2:0012.

7. Nyame TT, Lemon KP, Kolter R, Liao EC. High throughput assay for bacterial adhesion on acellular dermal matrices and synthetic surgical materials. Plast Reconstr Surg. 2011; 128:1061–1068.
8. Pandit S, Kim HJ, Song KY, Jeon JG. Relationship between fluoride concentration and activity against virulence factors and viability of a cariogenic biofilm: in vitro study. Caries Res. 2013; 47:539–547.

9. Chau NP, Pandit S, Jung JE, Jeon JG. Evaluation of Streptococcus mutans adhesion to fluoride varnishes and subsequent change in biofilm accumulation and acidogenicity. J Dent. 2014; 42:726–734.

10. Belli WA, Buckley DH, Marquis RE. Weak acid effects and fluoride inhibition of glycolysis by Streptococcus mutans GS-5. Can J Mi-crobiol. 1995; 41:785–791.
11. Pandit S, Cai JN, Song KY, Jeon JG. Identification of anti-biofilm components in Withania somnifera and their effect on virulence of Streptococcus mutans biofilms. J Appl Microbiol. 2015; 119:571–581.

12. Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000; 146:2395–2407.

13. Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005; 13:20–26.

14. Flemming HC, Neu TR, Wozniak DJ. The EPS matrix: the “house of biofilm cells”. J Bacteriol. 2007; 189:7945–7947.

15. Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010; 192:3024–3032.
Go to : 
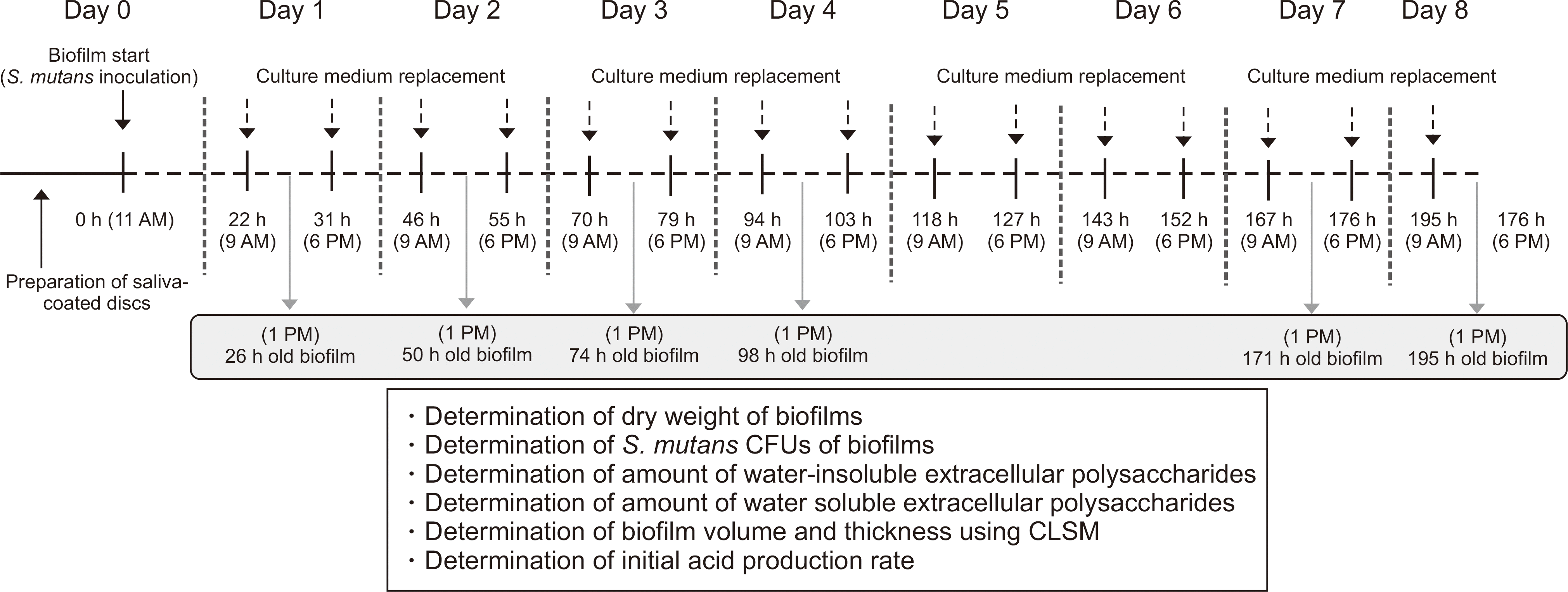 | Fig. 1.
S. mutans biofilm formation and experimental scheme for the present study. CFUs and CLSM represent colony forming units and confocal laser scanning microscope, respectively. |
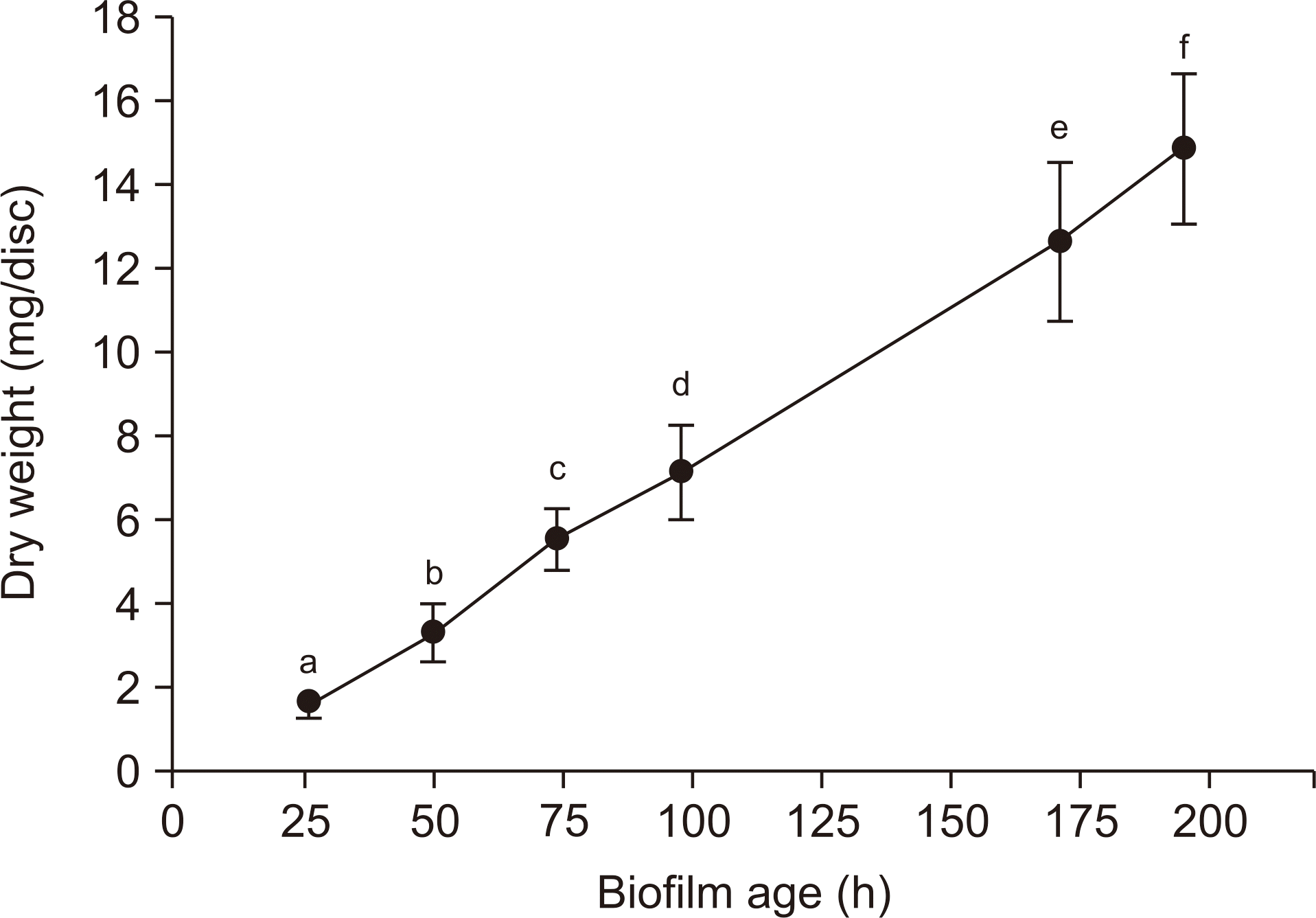 | Fig. 2.Change over time in dry weight of S. mutans biofilms. The data represent the mean±SD (n=8). Values followed by the same superscript are not significantly different from each other. |
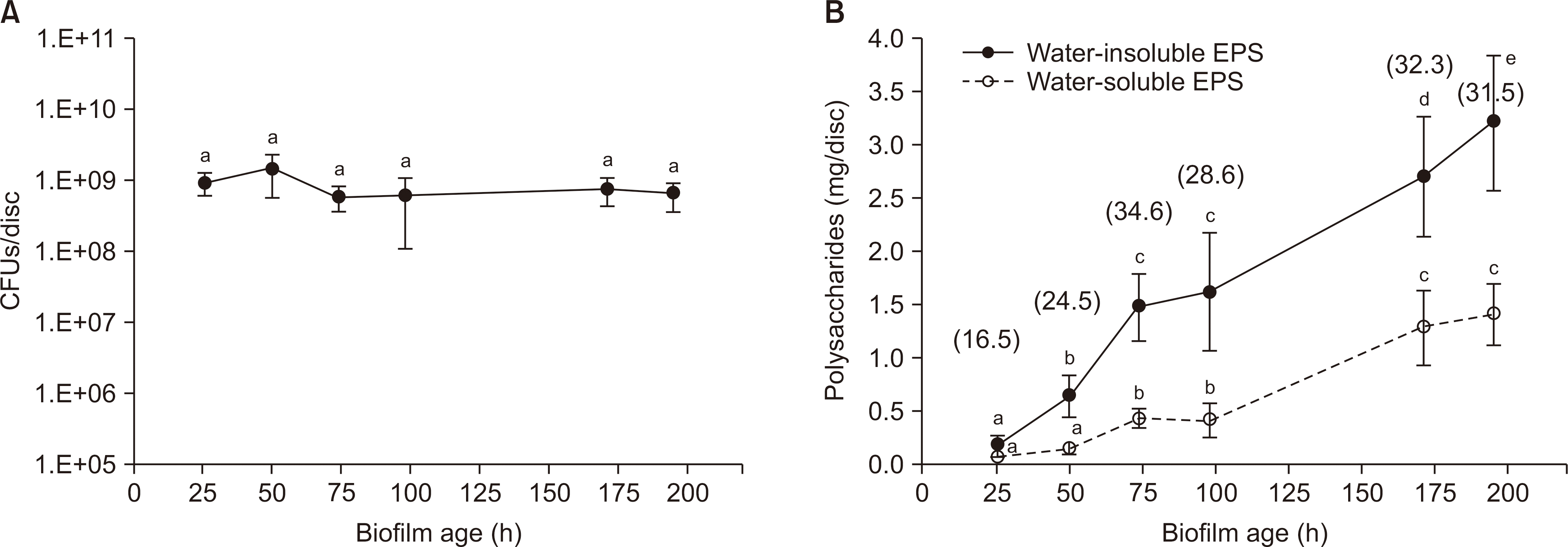 | Fig. 3.Change over time in CFUs (A) and extracelluar polysaccharides (EPS, B) of S. mutans biofilms. The numbers in parenthesis represent the percentage of water-insoluble and water-soluble polysaccharide out of dry weight. The data represent the mean±SD (n=8). Values followed by the same superscript are not significantly different from each other. |
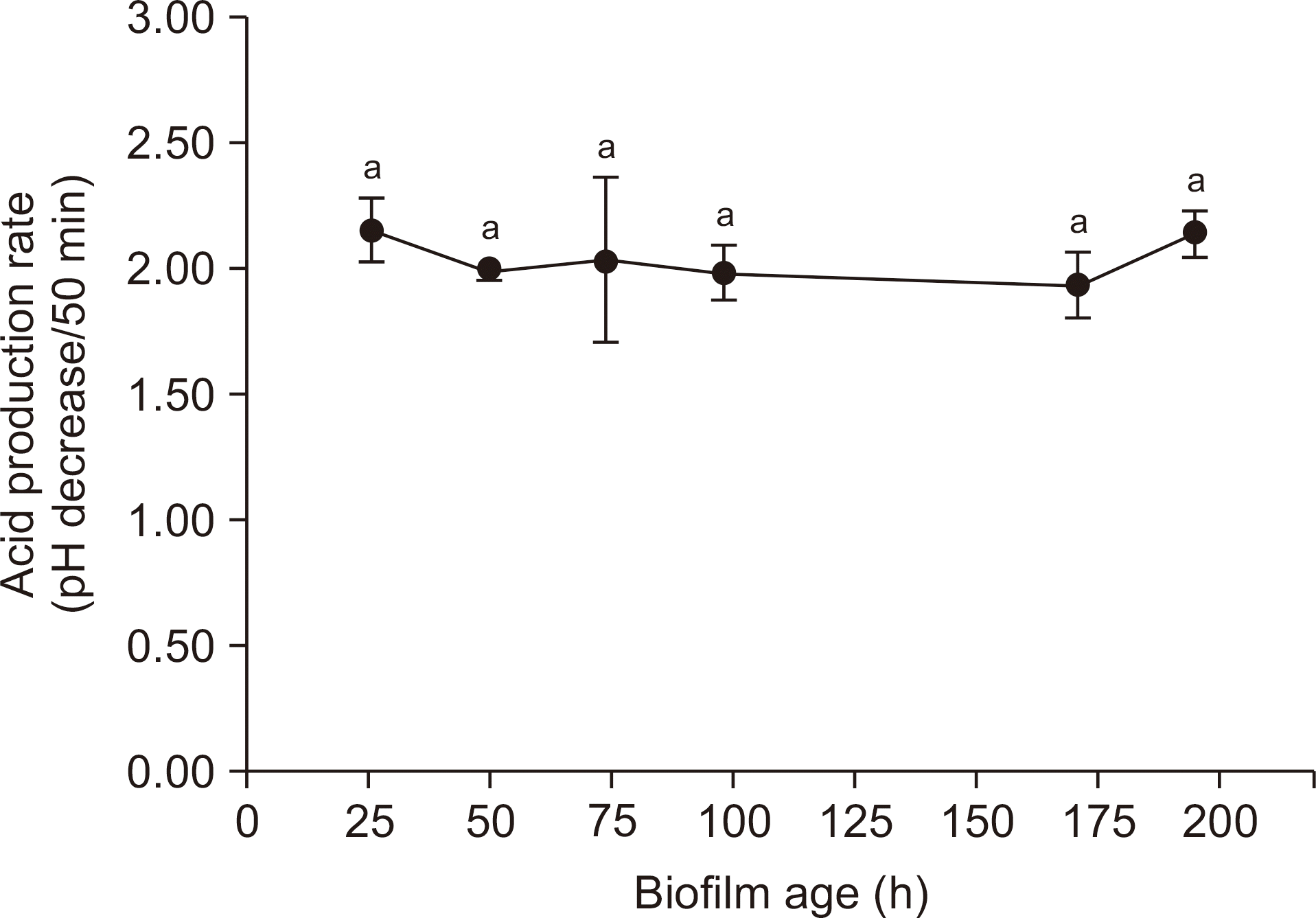 | Fig. 4.Changes over time in acidogenic ability of S. mutans biofilms. The data represent the mean±SD (n=8). Values followed by the same superscript are not significantly different from each other. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download