Abstract
Purpose
Skin grafts have been widely used in managing extensive chest wall defects after mastectomy for advanced breast cancer. However, their durability and tolerability to radiotherapy is still controversial. A thoracoabdominal (TA) flap with a few technical refinements can safely transfer a larger flap while minimizing complications.
Methods
From January 2007 to February 2018, a retrospective review was performed to compare 2 groups after wide breast excision: skin graft group (group 1) and lateral-based, single vertical incision rotation-advancement TA flap (group 2). Patients' demographics, operative details, complications, hospital stay, postoperative outpatient visits, cost, and start of adjuvant therapy were analyzed between the 2 groups.
Results
During the study period, 34 patients received skin graft and 41 patients received TA flap. group 2 had a shorter hospital stay (6.41 ± 2.64 days vs. 12.62 ± 4.60 days, P < 0.001) and shorter time to complete wound healing (29.27 ± 18.68 days vs. 39.24 ± 27.70 days, P = 0.03) than group 1. There was also a difference in the period from surgery to initiation of adjuvant therapy (group 1, 45.04 days ± 17.79 days; group 2, 37.07 ± 15.38 days, P = 0.073). Although limitation in shoulder motion was more frequent in group 2, limitation of motion for >1 year was observed in 4 patients in only group 1 (43.90% vs. 38.24%, P = 0.613).
Locally advanced breast cancer treatment involves radical mastectomy followed by adjuvant therapy. Because of local extension, mastectomy often requires a reconstructive procedure, with skin grafting being the simplest. However, skin grafting is not applicable when mastectomy involves underlying musculoskeletal resection. Moreover, the skin-grafted chest wall is fragile, particularly when adjuvant radiation is indicated. Various locoregional myocutaneous, fasciocutaneous, or dermofat flaps have been suggested for rapid healing and potentially curative cancer excision [1234]. Chest wall coverage following wide excision for locally advanced breast cancer is not a well-known topic compared with breast mound reconstruction, with no consensus yet. Since 2011, skin grafts have been replaced with locoregional thoracoabdominal (TA) flaps in most cases; medial- and lateral-based flaps were initially used according to the location and shape of the defect. Early analysis revealed that lateral-based flaps resulted in lesser complications, with a larger flap transferred safely [5]. We have applied a lateral-based, single vertical incision rotationadvancement TA flap for locally advanced or aggressive breast cancer and reported our technical refinements focusing on safely transferring a larger flap while minimizing complications.
This protocol was approved by the Institutional Review Board of Asan Medical Center (2014-1528) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. A retrospective review of the medical records identified 41 consecutive patients in whom lateral-based TA flaps were performed following wide excision of locally advanced breast cancer between June 2013 and February 2018. Patients' age, body mass index, comorbidities such as diabetes and hypertension, and smoking history data were collected. Operative factors such as operative time, defect size, mastectomy specimen weight, and history of transfusion were also analyzed. Mean hospital stay, period between operation and complete wound healing, number of outpatient clinic visits for postoperative wound care, and time interval from complete wound healing to initiation of adjuvant therapy were compared with those in patients who underwent conventional skin grafting between January 2007 and June 2015 (n = 34). Moreover, reports of postoperative pain and shoulder movement discomfort management by the rehabilitation team were obtained.
To analyze the differences between the 2 groups, Student t-test was used for continuous variables, and the Mann-Whitney U-test was used in small numbers of subjects. Categorical variables were evaluated using chi-square test and Fisher exact test. Shoulder motion limitation was assessed by univariate logistic regression. All statistical analyses were conducted using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA).
When skin and soft tissue defects develop, the upper and lower mastectomy flap is pulled gently to narrow the defect. Each flap is fixed in the pectoralis major muscle using an absorbable buried suture.
To cover the remaining defect, split-thickness skin is harvested from the medial thigh. The size of the harvested skin depends on the defect. The thickness of the skin is set to 0.012 in (0.3 mm). After harvesting, an epinephrine-soaked wet gauze is placed on the donor site for hemostasis. The skin graft is placed on the chest defect with a stapler fixation. Tie-over dressing or negative pressure wound therapy is applied, and donor site dressing is applied with polyurethane foam.
Tie-over dressing or vacuum dressing is removed between 4 and 6 days postoperatively. The skin graft donor site dressing is changed every 2–3 days.
Fasciocutaneous dissection begins upward from the upper margin of the defect to the clavicle. The skin and subcutaneous tissue mastectomy flap of the lower margin is also elevated from the anterior rectus sheath. Small soft tissue defects could be covered by bilateral advancement of these 2 flaps without additional incision, leaving only a transverse incision similar to that in conventional radical mastectomy. Closure starts from the medial side with dog ear management at the lateral side. If bilateral advancement is not sufficient in covering the defect without tension, a vertical curvilinear incision is designed, which starts from the most medial part of the defect to the umbilicus level (Fig. 1A, B). After a vertical incision is made, dissection is continued downward on the suprafascial layer. When the flap is elevated, as many perforators as possible from the lateral intercostal arteries are preserved for flap circulation, whereas superior epigastric perforators piercing the rectus sheath are mostly sacrificed to maximize flap mobility.
Upward flap mobilization (advancement) begins from the caudal side. Temporary flap medial margin suturing to the opposite side allows advancement. The distal tip of the flap is then laterally rotated to meet the upper flap at the upper margin center. TA and upper flap advancement transform the lateral defect into an oval shape, which is then approximated from the lateral side (Fig. 1B). Adjusting the advancement and rotation allows defect closure even after a few centimeters of the most distal tip is excised (Fig. 1C, D). The lateral dog ear is not immediately addressed in case of excessive closing tension to serve as a donor site for skin grafting intraoperatively or postoperatively.
Compromised circulation at the distal flap wound is recognized intraoperatively. A poorly perfused area should be discarded and managed accordingly. Slight adduction of fully abducted arm helps to mitigate excessive tension. Progressive tension sutures in the middle of the flap greatly help in releasing closing tension (Fig. 2A). A small full-thickness skin graft can be harvested from the lateral redundant tissue and inserted in case of excessive closing tension and/or compromised flap tip circulation (Fig. 2B–D).
The mean age of the skin graft group (group 1) and TA flap group (group 2) were 49.91 ± 11.65 and 45.15 ± 10.64 years, respectively (P = 0.068). The most common histologic findings were invasive ductal carcinoma (IDC) (group 1, 32 of 34; group 2, 35 of 41), myxofibrosarcoma, angiosarcoma, and spindle cell neoplasm. The mean defect size was 133.51 ± 98.73 cm2 in the skin graft group and 253.71 ± 136.08 cm2 (P < 0.001) in the TA flap group. The mastectomy weights were 706.02 ± 630.08 g and 834.86 ± 680.15 g in the skin graft and TA groups, respectively (P = 0.421). The mean duration of surgery was 77.82 ± 40.10 minutes in the skin graft group and 87.76 ± 52.15 minutes in the TA flap group (P = 0.472) (Table 1).
The mean duration of hospital stay was 12.62 ± 4.60 and 6.41 ± 2.64 days in groups 1 and 2, respectively (P < 0.001). Moreover, the time to complete wound healing postoperatively was 39.74 ± 27.70 days in the skin graft group and 29.27 ± 18.68 days in the TA flap group (P = 0.03). Although group 1 had more outpatient clinic visits than group 2, it was not statistically significantly different (2.82 ± 1.72 vs. 2.56 ± 2.05, P = 0.567). Regarding tissue necrosis, 15 patients (44.12%) in the skin graft group and 17 patients (42.5%) in the TA flap group showed a similar incidence rate; however, only 2 patients in the TA flap group required additional debridement and repair, whereas the skin graft group showed skin loss of >10% in 4 patients, who then required additional skin graft (P = 0.049). During radiation therapy, 6 (17.65%) and 3 patients (7.32%) had wound problems in the skin graft and TA flap groups, respectively. The skin graft group required an average of 45.04 days postoperatively for adjuvant therapy, whereas the TA flap group needed 29.27 days for postoperative treatment (P = 0.073) (Table 2).
The average costs of surgery in each group were 1,492,472 ± 845,129 Korean won (KRW) and 2,175,951 ± 960,579 KRW, in groups 1 and 2, respectively (P = 0.002). The hospitalization costs were 4,626,168 ± 1,901,620 KRW and 5,482,024 ± 2,414,046 KRW in the skin graft and TA flap groups, respectively, but there was no statistically significant difference (P = 0.104). In contrast, the expenditure on outpatient visits was 196,134 ± 100,214 KRW in the skin graft group and 116,753 ± 126,176 KRW in the TA flap group, in which the TA flap group had lesser expenditure, but the result was not statistically significant (P = 0.133) (Table 3).
Eight patients (23.53%) in the skin graft group and 7 patients (17.07%) in the TA flap group complained of shoulder pain, but no patients required opioids or long-term analgesics (P = 0.685). Thirteen patients in group 1 (38.24%) and 18 patients in group 2 (43.90%) underwent rehabilitation due to limitation of shoulder motion (P = 0.613). Four patients in the skin graft group had persistent motor function limitation of >1 year. In contrast, one patient in the TA flap group had no improvement and underwent additional release with Z-plasty within 12 months. No patient had shoulder movement disorder for >1 year (P = 0.047) (Table 4).
A 43-year-old woman presented with palpable mass on her right breast and was diagnosed with IDC (clinical stage T4N3M0, Fig. 3A). Neoadjuvant chemotherapy was administered with 4 cycles of adriamycin and cyclophosphamide (Fig. 3B), followed by 4 cycles of docetaxel (Fig. 3C). Preoperatively, she had residual IDC of stage 1A and underwent wide local excision (Fig. 3D) and reconstruction with single vertical incision TA flap. Adjuvant Herceptin and hormonal therapies were started on postoperative day 29. The patient also underwent adjuvant radiation therapy (50 Gy/25 fractions to the chest wall and 45 Gy/25 fractions to the supraclavicular lymph nodes, axilla, and posterior axillary boost, Fig. 3E) starting on postoperative day 41.
Advanced breast cancer often requires extensive excision, including a wide breast skin tissue. Soft tissue coverage is always problematic after wide-area resection. Reconstruction with normal tissues, such as the latissimus dorsi musculocutaneous flap, free deep inferior epigastric artery perforator flap, and free transverse rectus abdominis musculocutaneous flap, is robust, cosmetically beneficial, and much more tolerant in adjuvant chemotherapy and radiotherapy compared to skin grafting. Nevertheless, because most patients have a high pathologic stage, they are reluctant in receiving breast reconstruction. Therefore, in most patients, skin grafts have been used to cover the defect postoperatively. However, in the case of skin grafts, as the skin is thinner than the adjacent mastectomy tissues, severe distortion of the shape develops, and unless neovascularization occurs, it results in marginal necrosis requiring additional skin graft or prolonged wound healing. There is also high possibility of wound problems while receiving radiotherapy [6].
TA flaps date back to the late 1970s when medial- and lateral-based flaps were both described [34]. We prefer lateral-based flaps perfused by lateral intercostal vessels for several reasons. Medial-based flaps often fail to reach the most distal part of the defect or cause partial necrosis. Lateral-based TA flaps have robust circulation even with fewer vessels in a hatchet shape or transversely oriented design [78].
The skin graft and TA flap groups showed no significant difference in mastectomy weight and mean surgical time, but there was a difference in average defect size (skin graft group, 133.51 ± 98.73 cm2; TA flap group, 253.71 ± 136.08 cm2; P < 0.001). This difference was due to the timing of defect evaluation. When skin grafting was performed, defect size measurement was conducted after the approximation of the upper and lower flaps of the remaining mastectomy skin flap, whereas the TA flap defect was estimated not by full approximation but by neutral tension status.
The mean duration of hospital stay, postoperatively, was shorter in the TA flap group, and the TA flap group showed a shorter time interval from surgery to complete wound healing. Interestingly, the average surgical and hospitalization costs were lower in the skin graft group than in the TA flap group. There are 2 possible reasons for the result. The first reason is that the cost of the fasciocutaneous flap (713,140 KRW, as of 2019) is higher than that of the skin graft (350,300 KRW, as of 2019). The second reason is the timing of each surgery. The skin graft group was analyzed in the period from 2007 to 2015, and the TA flap group in the period from 2013 to 2018. Therefore, the actual cost will be different from that in the results, since the data are based on an absolute number without considering inflation rates.
There were more frequent visits to the plastic surgery outpatient clinic for wound management postoperatively in the skin graft group; however, these were not statistically significantly different. Moreover, the skin graft group had higher expenditure in total outpatient visits.
The incidence of postoperative tissue necrosis was not different between the 2 groups. However, only 2 patients in the TA flap group required debridement and repair, while 4 patients in the skin graft group had skin loss of ≥10%. In regard to new wound problems during radiation therapy, there were 6 and 3 patients with wound problem in the skin graft and TA flap groups, respectively. Although the incidence rate doubled, it did not show a statistically significant difference. This is probably due to the small number of patients in the analysis group.
After extensive resection of advanced breast cancer, there may be development of shoulder pain and mobility impairment due to contracture after the wound healing process. The pain and limitation of motion ratios were slightly higher in the skin graft group than in the TA flap group, but there was no statistically significant difference. In both groups, no opioid was needed, and all pain symptoms disappeared within 6 months. Thirteen (38.24%) and 18 patients (43.90%) in the skin graft and TA flap groups, respectively, were treated with rehabilitation medicine due to limitation of shoulder motion. However, 4 patients in the skin graft group had >1 year of persistent restriction of movement. This suggests that movement limitation in the TA flap group may occur at an early stage, but stretching ability of the fasciocutaneous flap is better than that of the skin graft. One patient in the TA flap group underwent release with Z-plasty, but the defect size was extremely large (30 cm × 23 cm).
A typical defect after locally advanced breast cancer mastectomy usually involves the whole breast footprint. In this situation, a skin graft is a generally considered the choice for reconstruction. However, unreliable durability, wound problems during radiotherapy, and large donor site scar, which will remain in the harvest site permanently, are disadvantages in practical and cosmetic aspects.
Our single vertical incision TA flap has a consistent, simple, and straightforward design and involves rotation-advancement tissue mobilization, which minimizes concerns involving the donor site. This technique does not involve sophisticated procedures such as perforating vessel identification or pedicle skeletonization. It can also be re-elevated and further advanced when local recurrence of the tumor occurs at the margin of the flap. This flap can be also applied to any unilateral large chest wall skin and soft tissue defect such as radiation necrosis.
Our technique does not transfer a flap of the same size as the defect. We transfer a single rotation-advancement flap of the maximum size and mobilize tissue from the upper and lateral parts of the defect. As a result, the highest point of the closing line usually lies far inferior to the initial defect (Fig. 4A–D). Vieira et al. [8] described a TA horizontal flap that transferred similar tissue as ours and mobilized tissue from the upper and medial areas.
Moreover, we have suggested some technical refinements in transferring the maximal size of flap while minimizing perfusion- and tension-related complications. As a result, we have shown the oncological and economic benefits of our flap over skin grafts by comparing the duration of hospital stay and start of adjuvant therapy.
This surgery is not an aesthetic reconstruction and intends to achieve locoregional disease control. However, future chance of breast reconstruction should be considered [9]. Reconstruction based on a tissue expander would be applicable with fewer surgeries and complications compared with skin grafts or other complex flaps, although lower abdominal flap use is precluded. A latissimus flap would still be available whenever necessary, which is one reason we prefer a cutaneous flap to a musculocutaneous one.
This study has some limitations. First, the number of cases is relatively small. Moreover, the operative time of the 2 groups is somewhat serial, which may cause a bias in the interpretation of operation, hospital, and outpatient costs. Furthermore, the type of dressing used in the treatment of the patients is not the same as those in the past. We assume that a well-designed large-scale study in the future will reinforce the aforementioned results.
Therefore, our lateral-based single vertical incision TA flap has a consistent, simple, straightforward design and involves rotation-advancement tissue mobilization, which minimizes concerns involving the donor site. This technique does not involve sophisticated procedures such as perforating vessel identification or pedicle skeletonization. It not only is simple but also yields a superior outcome compared to a conventional skin graft. TA flap promises rapid recovery and adjuvant therapy for patients, improving quality of life and increasing survival rates.
Figures and Tables
Fig. 1
Flap design and tissue mobilization. (A) Schematic presenting directions of tissue mobilization. (B) Design of the single vertical incision thoracoabdominal flap; schematic presenting the lateral intercostal, superior epigastric, and deep inferior epigastric perforating vessels. (C) Immediate postoperative image. (D) Intraoperative image after locally advanced breast cancer mastectomy.

Fig. 2
Measures to prevent tension-related complications. (A) A tension-releasing suture was applied at the middle of the flap between Scarpa's fascia of the flap and anterior rectus sheath (arrow). (B) Lateral redundant tissue (arrow) could be used as a donor site for skin grafting in case of excessive closing tension or compromised flap tip circulation. (C) A small full-thickness skin graft was inserted (arrow) due to excessive closing tension. (D) Two months after completion of adjuvant radiation therapy of patient in panel C.

Fig. 3
Clinical images of the representative case. (A) A 43-year-old patient diagnosed with invasive ductal carcinoma (cT4dN3M0). (B) At the end of 4 cycles of neoadjuvant chemotherapy with adriamycin and cyclophosphamide. (C) After another 4 cycles of docetaxel, just before surgery. (D) Defect after wide excision of locally advanced breast cancer after neoadjuvant chemotherapy. (E) Adjuvant radiation therapy was started on postoperative day 41.
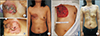
Fig. 4
Rotation-advancement flap transfer and mobilization of the tissue from the upper and lateral parts resulted in a closing line at the center of the initial defect. (A) A 34-year-old patient diagnosed with invasive micropapillary carcinoma (T3N3M0) during adjuvant radiation therapy. (B) A 32-year-old patient with IDC (T4N2M1) after completion of adjuvant radiation therapy. (C) A 43-year-old patient with IDC (T3N0M0) after completion of adjuvant radiation therapy. (D) A 67-year-old patient with myxofibrosarcoma, 1 month postoperatively. IDC, invasive ductal carcinoma.
References
1. Woo E, Tan BK, Koong HN, Yeo A, Chan MY, Song C. Use of the extended V-Y latissimus dorsi myocutaneous flap for chest wall reconstruction in locally advanced breast cancer. Ann Thorac Surg. 2006; 82:752–755.

2. Charanek AM. A bilobed thoracoabdominal myocutaneous flap for large thoracic defects. Ann Plast Surg. 2014; 72:451–456.

3. Davis WM, McCraw JB, Carraway JH. Use of a direct, transverse, thoracoabdominal flap to close difficult wounds of the thorax and upper extremity. Plast Reconstr Surg. 1977; 60:526–533.

4. Baroudi R, Pinotti JA, Keppke EM. A transverse thoracoabdominal skin flap for closure after radical mastectomy. Plast Reconstr Surg. 1978; 61:547–554.

5. Park JS, Ahn SH, Son BH, Kim EK. Using local flaps in a chest wall reconstruction after mastectomy for locally advanced breast cancer. Arch Plast Surg. 2015; 42:288–294.

6. Bui DT, Chunilal A, Mehrara BJ, Disa JJ, Alektiar KM, Cordeiro PG. Outcome of split-thickness skin grafts after external beam radiotherapy. Ann Plast Surg. 2004; 52:551–556.

7. Persichetti P, Brunetti B, Cagli B, Tenna S. Chest wall reconstruction with the perforator-plus thoracoabdominal flap. Plast Reconstr Surg. 2012; 130:488e–489e.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download