1. Steen VD, Conte C, Owens GR, Medsger TA Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994; 37:1283–1289. PMID:
7945490.

2. Fewins HE, McGowan I, Whitehouse GH, Williams J, Mallya R. High definition computed tomography in rheumatoid arthritis associated pulmonary disease. Br J Rheumatol. 1991; 30:214–216. PMID:
2049584.

3. Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997; 156(2 Pt 1):528–535. PMID:
9279235.

4. McDonagh J, Greaves M, Wright AR, Heycock C, Owen JP, Kelly C. High resolution computed tomography of the lungs in patients with rheumatoid arthritis and interstitial lung disease. Br J Rheumatol. 1994; 33:118–122. PMID:
8162474.

5. Cortet B, Perez T, Roux N, Flipo RM, Duquesnoy B, Delcambre B, et al. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis. 1997; 56:596–600. PMID:
9389220.

6. Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014; 146:422–436. PMID:
24576924.
7. Cavagna L, Monti S, Grosso V, Boffini N, Scorletti E, Crepaldi G, et al. The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. Biomed Res Int. 2013; 2013:759760. PMID:
24205507.

8. de Lauretis A, Veeraraghavan S, Renzoni E. Review series: aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis. 2011; 8:53–82. PMID:
21339375.

9. Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010; 62:1583–1591. PMID:
20155830.

10. Assayag D, Lubin M, Lee JS, King TE, Collard HR, Ryerson CJ. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology. 2014; 19:493–500. PMID:
24372981.

11. Saag KG, Kolluri S, Koehnke RK, Georgou TA, Rachow JW, Hunninghake GW, et al. Rheumatoid arthritis lung disease: determinants of radiographic and physiologic abnormalities. Arthritis Rheum. 1996; 39:1711–1719. PMID:
8843862.

12. Turesson C. Extra-articular rheumatoid arthritis. Curr Opin Rheumatol. 2013; 25:360–366. PMID:
23425964.

13. Doyle TJ, Lee JS, Dellaripa PF, Lederer JA, Matteson EL, Fischer A, et al. A roadmap to promote clinical and translational research in rheumatoid arthritis-associated interstitial lung disease. Chest. 2014; 145:454–463. PMID:
24590021.

14. Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics: a large multicentre UK study. Rheumatology (Oxford). 2014; 53:1676–1682. PMID:
24758887.
15. Ytterberg AJ, Joshua V, Reynisdottir G, Tarasova NK, Rutishauser D, Ossipova E, et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis. 2015; 74:1772–1777. PMID:
24817415.

16. Aubart F, Crestani B, Nicaise-Roland P, Tubach F, Bollet C, Dawidowicz K, et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol. 2011; 38:979–982. PMID:
21362759.

17. Luukkainen R, Saltyshev M, Pakkasela R, Nordqvist E, Huhtala H, Hakala M. Relationship of rheumatoid factor to lung diffusion capacity in smoking and non-smoking patients with rheumatoid arthritis. Scand J Rheumatol. 1995; 24:119–120. PMID:
7747143.

18. Tuomi T, Heliovaara M, Palosuo T, Aho K. Smoking, lung function, and rheumatoid factors. Ann Rheum Dis. 1990; 49:753–756. PMID:
2241263.

19. Lamblin C, Bergoin C, Saelens T, Wallaert B. Interstitial lung diseases in collagen vascular diseases. Eur Respir J Suppl. 2001; 32:69s–80s. PMID:
11816826.
20. Desai SR, Veeraraghavan S, Hansell DM, Nikolakopolou A, Goh NS, Nicholson AG, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology. 2004; 232:560–567. PMID:
15286324.

21. Walker UA, Tyndall A, Czirjak L, Denton C, Farge-Bancel D, Kowal-Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007; 66:754–763. PMID:
17234652.

22. Zhang XJ, Bonner A, Hudson M, Baron M, Pope J. Canadian Scleroderma Research Group. Association of gastroesophageal factors and worsening of forced vital capacity in systemic sclerosis. J Rheumatol. 2013; 40:850–858. PMID:
23547215.

23. Wells AU, Margaritopoulos GA, Antoniou KM, Denton C. Interstitial lung disease in systemic sclerosis. Semin Respir Crit Care Med. 2014; 35:213–221. PMID:
24668536.

24. Briggs DC, Vaughan RW, Welsh KI, Myers A, duBois RM, Black CM. Immunogenetic prediction of pulmonary fibrosis in systemic sclerosis. Lancet. 1991; 338:661–662. PMID:
1679476.

25. Hu PQ, Fertig N, Medsger TA Jr, Wright TM. Correlation of serum anti-DNA topoisomerase I antibody levels with disease severity and activity in systemic sclerosis. Arthritis Rheum. 2003; 48:1363–1373. PMID:
12746909.

26. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011; 183:788–824. PMID:
21471066.
27. Solomon JJ, Olson AL, Fischer A, Bull T, Brown KK, Raghu G. Scleroderma lung disease. Eur Respir Rev. 2013; 22:6–19. PMID:
23457159.

28. Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002; 165:1581–1586. PMID:
12070056.

29. Moore OA, Goh N, Corte T, Rouse H, Hennessy O, Thakkar V, et al. Extent of disease on high-resolution computed tomography lung is a predictor of decline and mortality in systemic sclerosis-related interstitial lung disease. Rheumatology (Oxford). 2013; 52:155–160. PMID:
23065360.

30. Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007; 66:940–944. PMID:
17329309.

31. Dellaripa PF, Fischer A, Flaherty KR. Pulmonary manifestations of rheumatic disease: a comprehensive guide. New York: Springer;2014.
32. Palm O, Garen T, Berge Enger T, Jensen JL, Lund MB, Aalokken TM, et al. Clinical pulmonary involvement in primary Sjogren's syndrome: prevalence, quality of life and mortality: a retrospective study based on registry data. Rheumatology (Oxford). 2013; 52:173–179. PMID:
23192906.
33. Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, Del Pino-Montes J, et al. Primary Sjogren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore). 2008; 87:210–219. PMID:
18626304.
34. Yazisiz V, Arslan G, Ozbudak IH, Turker S, Erbasan F, Avci AB, et al. Lung involvement in patients with primary Sjogren's syndrome: what are the predictors? Rheumatol Int. 2010; 30:1317–1324. PMID:
19844720.
35. Uffmann M, Kiener HP, Bankier AA, Baldt MM, Zontsich T, Herold CJ. Lung manifestation in asymptomatic patients with primary Sjogren syndrome: assessment with high resolution CT and pulmonary function tests. J Thorac Imaging. 2001; 16:282–289. PMID:
11685093.
36. Cain HC, Noble PW, Matthay RA. Pulmonary manifestations of Sjogren's syndrome. Clin Chest Med. 1998; 19:687–699. PMID:
9917960.
37. Parambil JG, Myers JL, Lindell RM, Matteson EL, Ryu JH. Interstitial lung disease in primary Sjogren syndrome. Chest. 2006; 130:1489–1495. PMID:
17099028.
38. Sirianni FE, Milaninezhad A, Chu FS, Walker DC. Alteration of fibroblast architecture and loss of Basal lamina apertures in human emphysematous lung. Am J Respir Crit Care Med. 2006; 173:632–638. PMID:
16415275.

39. Sharp GC, Irvin WS, Tan EM, Gould RG, Holman HR. Mixed connective tissue disease: an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972; 52:148–159. PMID:
4621694.
40. Tani C, Carli L, Vagnani S, Talarico R, Baldini C, Mosca M, et al. The diagnosis and classification of mixed connective tissue disease. J Autoimmun. 2014; 48-49:46–49. PMID:
24461387.

41. Gunnarsson R, Aalokken TM, Molberg O, Lund MB, Mynarek GK, Lexberg AS, et al. Prevalence and severity of interstitial lung disease in mixed connective tissue disease: a nationwide, cross-sectional study. Ann Rheum Dis. 2012; 71:1966–1972. PMID:
22550317.

42. Gunnarsson R, El-Hage F, Aalokken TM, Reiseter S, Lund MB, Garen T, et al. Associations between anti-Ro52 antibodies and lung fibrosis in mixed connective tissue disease. Rheumatology (Oxford). 2016; 55:103–108. PMID:
26320136.

43. Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004; 63:297–301. PMID:
14962966.

44. Lee CS, Chen TL, Tzen CY, Lin FJ, Peng MJ, Wu CL, et al. Idiopathic inflammatory myopathy with diffuse alveolar damage. Clin Rheumatol. 2002; 21:391–396. PMID:
12223988.

45. Chen IJ, Jan Wu YJ, Lin CW, Fan KW, Luo SF, Ho HH, et al. Interstitial lung disease in polymyositis and dermatomyositis. Clin Rheumatol. 2009; 28:639–646. PMID:
19247576.

46. Hayashi S, Tanaka M, Kobayashi H, Nakazono T, Satoh T, Fukuno Y, et al. High-resolution computed tomography characterization of interstitial lung diseases in polymyositis/dermatomyositis. J Rheumatol. 2008; 35:260–269. PMID:
18085731.
47. Yu KH, Wu YJ, Kuo CF, See LC, Shen YM, Chang HC, et al. Survival analysis of patients with dermatomyositis and polymyositis: analysis of 192 Chinese cases. Clin Rheumatol. 2011; 30:1595–1601. PMID:
21915609.
48. Ji SY, Zeng FQ, Guo Q, Tan GZ, Tang HF, Luo YJ, et al. Predictive factors and unfavourable prognostic factors of interstitial lung disease in patients with polymyositis or dermatomyositis: a retrospective study. Chin Med J (Engl). 2010; 123:517–522. PMID:
20367973.
49. Matsushita T, Hasegawa M, Fujimoto M, Hamaguchi Y, Komura K, Hirano T, et al. Clinical evaluation of anti-aminoacyl tRNA synthetase antibodies in Japanese patients with dermatomyositis. J Rheumatol. 2007; 34:1012–1018. PMID:
17309126.
50. Yoshifuji H, Fujii T, Kobayashi S, Imura Y, Fujita Y, Kawabata D, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006; 39:233–241. PMID:
16769657.

51. Tillie-Leblond I, Wislez M, Valeyre D, Crestani B, Rabbat A, Israel-Biet D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax. 2008; 63:53–59. PMID:
17557770.

52. Kono M, Nakamura Y, Enomoto N, Hashimoto D, Fujisawa T, Inui N, et al. Usual interstitial pneumonia preceding collagen vascular disease: a retrospective case control study of patients initially diagnosed with idiopathic pulmonary fibrosis. PLoS One. 2014; 9:e94775. PMID:
24736601.

53. Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol. 2011; 37:100–109. PMID:
21390438.
54. Doyle TJ, Dellaripa PF. Lung manifestations in the rheumatic diseases. Chest. 2017; 152:1283–1295. PMID:
28552544.

55. Mittoo S, Fell CD. Pulmonary manifestations of systemic lupus erythematosus. Semin Respir Crit Care Med. 2014; 35:249–254. PMID:
24668539.

56. Chua F, Higton AM, Colebatch AN, O'Reilly K, Grubnic S, Vlahos I, et al. Idiopathic inflammatory myositis-associated interstitial lung disease: ethnicity differences and lung function trends in a British cohort. Rheumatology (Oxford). 2012; 51:1870–1876. PMID:
22763991.

57. Eisenberg H, Dubois EL, Sherwin RP, Balchum OJ. Diffuse interstitial lung disease in systemic lupus erythematosus. Ann Intern Med. 1973; 79:37–45. PMID:
4124484.

58. ter Borg EJ, Groen H, Horst G, Limburg PC, Wouda AA, Kallenberg CG. Clinical associations of antiribonucleoprotein antibodies in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 1990; 20:164–173. PMID:
2287941.

59. Ward MM, Polisson RP. A meta-analysis of the clinical manifestations of older-onset systemic lupus erythematosus. Arthritis Rheum. 1989; 32:1226–1232. PMID:
2803325.

60. Perez T, Farre JM, Gosset P, Wallaert B, Duquesnoy B, Voisin C, et al. Subclinical alveolar inflammation in rheumatoid arthritis: superoxide anion, neutrophil chemotactic activity and fibronectin generation by alveolar macrophages. Eur Respir J. 1989; 2:7–13. PMID:
2540025.
61. Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012; 185:1147–1153. PMID:
22366047.
62. Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008; 168:159–166. PMID:
18227362.

63. Launay D, Remy-Jardin M, Michon-Pasturel U, Mastora I, Hachulla E, Lambert M, et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J Rheumatol. 2006; 33:1789–1801. PMID:
16960939.
64. Cottin V. Idiopathic interstitial pneumonias with connective tissue diseases features: a review. Respirology. 2016; 21:245–258. PMID:
26212251.

65. Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016; 352:h6819. PMID:
26912511.

66. Cottin V. Significance of connective tissue diseases features in pulmonary fibrosis. Eur Respir Rev. 2013; 22:273–280. PMID:
23997055.

67. Mosca M, Tani C, Bombardieri S. A case of undifferentiated connective tissue disease: is it a distinct clinical entity? Nat Clin Pract Rheumatol. 2008; 4:328–332. PMID:
18414458.

68. Kinder BW, Collard HR, Koth L, Daikh DI, Wolters PJ, Elicker B, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med. 2007; 176:691–697. PMID:
17556720.
69. Corte TJ, Copley SJ, Desai SR, Zappala CJ, Hansell DM, Nicholson AG, et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur Respir J. 2012; 39:661–668. PMID:
21920896.

70. Nunes H, Schubel K, Piver D, Magois E, Feuillet S, Uzunhan Y, et al. Nonspecific interstitial pneumonia: survival is influenced by the underlying cause. Eur Respir J. 2015; 45:746–755. PMID:
25537566.

71. Moua T, Zamora Martinez AC, Baqir M, Vassallo R, Limper AH, Ryu JH. Predictors of diagnosis and survival in idiopathic pulmonary fibrosis and connective tissue disease-related usual interstitial pneumonia. Respir Res. 2014; 15:154. PMID:
25472884.

72. Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med. 2007; 175:705–711. PMID:
17218621.
73. Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010; 35:1322–1328. PMID:
19996193.

74. Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004; 44:585–596. PMID:
15186274.

75. Kim DS. Interstitial lung disease in rheumatoid arthritis: recent advances. Curr Opin Pulm Med. 2006; 12:346–353. PMID:
16926650.

76. Cottin V, Thivolet-Bejui F, Reynaud-Gaubert M, Cadranel J, Delaval P, Ternamian PJ, et al. Interstitial lung disease in amyopathic dermatomyositis, dermatomyositis and polymyositis. Eur Respir J. 2003; 22:245–250. PMID:
12952255.

77. Douglas WW, Tazelaar HD, Hartman TE, Hartman RP, Decker PA, Schroeder DR, et al. Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med. 2001; 164:1182–1185. PMID:
11673206.

78. Cottin V. Pragmatic prognostic approach of rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010; 35:1206–1208. PMID:
20513909.

79. Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012; 380:689–698. PMID:
22901890.

80. Antin-Ozerkis D, Rubinowitz A, Evans J, Homer RJ, Matthay RA. Interstitial lung disease in the connective tissue diseases. Clin Chest Med. 2012; 33:123–149. PMID:
22365251.

81. Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009; 136:23–30. PMID:
19255290.

82. Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 2010; 138:251–256. PMID:
20682528.
83. Devouassoux G, Cottin V, Liote H, Marchand E, Frachon I, Schuller A, et al. Characterisation of severe obliterative bronchiolitis in rheumatoid arthritis. Eur Respir J. 2009; 33:1053–1061. PMID:
19129282.

84. Suda T, Kaida Y, Nakamura Y, Enomoto N, Fujisawa T, Imokawa S, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. 2009; 103:846–853. PMID:
19181509.

85. Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008; 63 Suppl 5:v1–v58. PMID:
18757459.
86. Wallace B, Vummidi D, Khanna D. Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol. 2016; 28:236–245. PMID:
27027811.
87. Aggarwal R, Oddis CV. Therapeutic advances in myositis. Curr Opin Rheumatol. 2012; 24:635–641. PMID:
22955021.

88. Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006; 54:3962–3970. PMID:
17133610.

89. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006; 354:2655–2666. PMID:
16790698.
90. Fischer A, Brown KK, Du Bois RM, Frankel SK, Cosgrove GP, Fernandez-Perez ER, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013; 40:640–646. PMID:
23457378.

91. Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016; 4:708–719. PMID:
27469583.
92. Kurita T, Yasuda S, Amengual O, Atsumi T. The efficacy of calcineurin inhibitors for the treatment of interstitial lung disease associated with polymyositis/dermatomyositis. Lupus. 2015; 24:3–9. PMID:
25297551.

93. Saunders P, Tsipouri V, Keir GJ, Ashby D, Flather MD, Parfrey H, et al. Rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease (RECITAL): study protocol for a randomised controlled trial. Trials. 2017; 18:275. PMID:
28619061.

94. Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007; 176:1026–1034. PMID:
17717203.

95. Ando K, Motojima S, Doi T, Nagaoka T, Kaneko N, Aoshima M, et al. Effect of glucocorticoid monotherapy on pulmonary function and survival in Japanese patients with scleroderma-related interstitial lung disease. Respir Investig. 2013; 51:69–75.

96. Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011; 378:498–506. PMID:
21777972.

97. Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford). 2010; 49:271–280. PMID:
19447770.

98. Lam GK, Hummers LK, Woods A, Wigley FM. Efficacy and safety of etanercept in the treatment of scleroderma-associated joint disease. J Rheumatol. 2007; 34:1636–1637. PMID:
17611970.
99. Launay D, Savale L, Berezne A, Le Pavec J, Hachulla E, Mouthon L, et al. Lung and heart-lung transplantation for systemic sclerosis patients: a monocentric experience of 13 patients, review of the literature and position paper of a multidisciplinary Working Group. Presse Med. 2014; 43(10 Pt 2):e345–e363. PMID:
25027464.

100. Assayag D, Lee JS, King TE Jr. Rheumatoid arthritis associated interstitial lung disease: a review. Medicina (B Aires). 2014; 74:158–165. PMID:
24736263.
101. Rojas-Serrano J, Gonzalez-Velasquez E, Mejia M, Sanchez-Rodriguez A, Carrillo G. Interstitial lung disease related to rheumatoid arthritis: evolution after treatment. Reumatol Clin. 2012; 8:68–71. PMID:
22341526.

102. Matteson EL, Bongartz T, Ryu JH, Crowson CS, Hartman TE, Dellaripa PF. Open-label, pilot study of the safety and clinical effects of rituximab in patients with rheumatoid arthritis-associated interstitial pneumonia. Open J Rheumatol Autoimmune Dis. 2012; 2:53–58.

103. Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, et al. Rituximab in severe, treatmentrefractory interstitial lung disease. Respirology. 2014; 19:353–359. PMID:
24286447.

104. Chartrand S, Swigris JJ, Peykova L, Fischer A. Rituximab for the treatment of connective tissue disease-associated interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2016; 32:296–304. PMID:
26847096.
105. Cohen JM, Miller A, Spiera H. Interstitial pneumonitis complicating rheumatoid arthritis. Sustained remission with azathioprine therapy. Chest. 1977; 72:521–524. PMID:
908223.
106. Mera-Varela A, Perez-Pampin E. Abatacept therapy in rheumatoid arthritis with interstitial lung disease. J Clin Rheumatol. 2014; 20:445–446. PMID:
25417684.

107. Mohr M, Jacobi AM. Interstitial lung disease in rheumatoid arthritis: response to IL-6R blockade. Scand J Rheumatol. 2011; 40:400–401. PMID:
21961704.

108. Dellaripa PF, Fry TA, Willoughby J, Arndt WF, Angelakis EJ, Campagna AC. The treatment of interstitial lung disease associated with rheumatoid arthritis with inflixima. Chest. 2003; 124:109S.

109. Marigliano B, Soriano A, Margiotta D, Vadacca M, Afeltra A. Lung involvement in connective tissue diseases: a comprehensive review and a focus on rheumatoid arthritis. Autoimmun Rev. 2013; 12:1076–1084. PMID:
23684699.

110. Ito I, Nagai S, Kitaichi M, Nicholson AG, Johkoh T, Noma S, et al. Pulmonary manifestations of primary Sjogren's syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med. 2005; 171:632–638. PMID:
15579729.
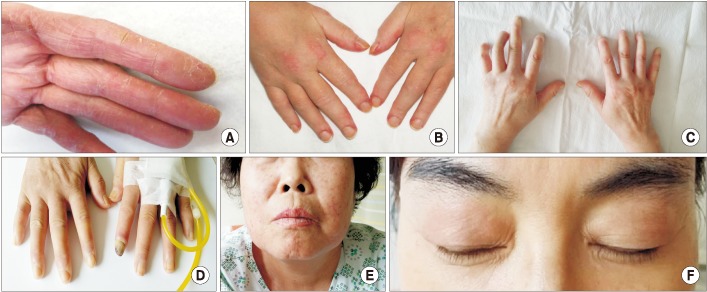
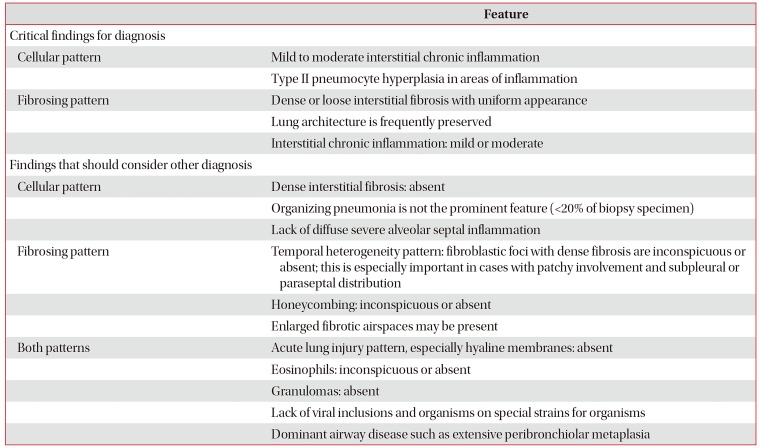
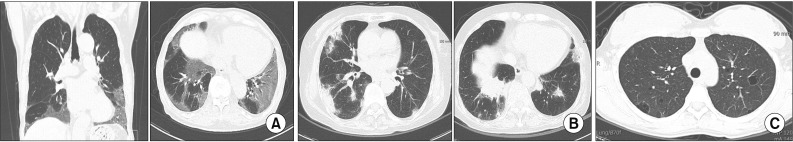
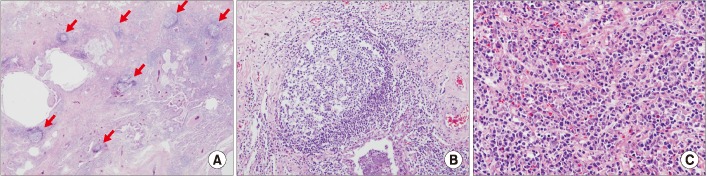





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download