This article has been
cited by other articles in ScienceCentral.
Abstract
Background
C-reactive protein (CRP) is an acute-phase protein whose level increases in response to tissue injury, infection, or other inflammation. It is used in clinical and forensic settings. Point-of-care (POC) testing has recently become available, and it is considered to be useful during postmortem examinations. However, laboratory testing of postmortem blood samples is difficult due to hemolysis and postmortem clotting.
Methods
The utility of POC testing for CRP during postmortem examination was evaluated using cardiac blood from the inferior vena cava. The whole blood sample was immediately tested using the POC instrument. Subsequently, the same sample was processed to obtain the serum, which was tested using common laboratory instruments.
Results
The postmortem POC test had a high positive predictive value and specificity, and the results strongly correlated with the laboratory test results.
Conclusion
POC CRP testing is valid in postmortem examination and can be used in forensic medicine (postmortem inspection and autopsy).
Keywords: Forensic Science, Forensic Medicine, C-Reactive Protein, Point-of-Care Testing
INTRODUCTION
C-reactive protein (CRP) is an acute-phase protein that is secreted from the liver in response to tissue injury, infection, or other inflammation.
1 It is used as a marker for inflammation in clinical and forensic medicine.
2 Although postmortem CRP level is lower than the corresponding antemortem level, it remains stable for a postmortem interval (PMI) of 31 days. CRP testing is used in routine autopsy practice to detect inflammatory disease.
23 Recent technological advances have facilitated point-of-care (POC) testing,
4 which provides an immediate result at the bedside, and in addition, it is considered to be useful for postmortem examination, facilitating decision-making during an autopsy and diagnosis of serious inflammation. According to the POC CRP test results, the forensic pathologist can change the autopsy technique and conduct additional postmortem tests such as histologic examination and bacterial culture. However, laboratory testing of postmortem blood samples is difficult due to hemolysis and postmortem clotting. Therefore, the present study aimed to confirm the possibility of POC testing for CRP and evaluate the forensic application of POC CRP tests.
METHODS
Subjects
The present study evaluated 85 forensic autopsy cases that were encountered between March and August 2017. During routine autopsy procedure, the pericardium was incised, and cardiac blood samples were obtained from the inferior vena cava (IVC) using a syringe; these samples were used for analysis.
Analytic methods for evaluation
The utility of POC CRP testing was evaluated using several analytic methods. The first method involved examining CRP levels in the cardiac whole blood during autopsy using a portable Alere AfinionTM AS100 Analyzer (Axis-Shield PoC AS, Oslo, Norway) which employs a solid phase immunochemical assay. This POC test can measure CRP values of 0.5–20 mg/dL within 4 minutes using a 1.5 µL blood sample, which is automatically corrected for hematocrit levels of 20%–60%. In the second method, antemortem and postmortem CRP results were compared in cases where both datasets were available. Antemortem CRP results were collected from medical records made before death. The time from death to autopsy was defined as the PMI, and the time from the antemortem test to autopsy was defined as the testing postmortem interval (tPMI). In the third method, the same cardiac blood specimen was centrifuged in serum separating tubes for 10 minutes at 3,000 rpm. The serum was subsequently tested using two widely used laboratory instruments: BT1500 (Biotecnica Instrument S.p.A., Rome, Italy) and Cobas 8000 (Roche Diagnostics, Indianapolis, IN, USA). These instruments use an immunoassay method. The CRP results from the POC testing and the laboratory instruments were compared.
Statistical analysis
All statistical analyses were performed using IBM SPSS for Windows (ver. 23.0; IBM Corp., Armonk, NY, USA). The general characteristics of the patients were reported as mean or number (%). The demographic characteristics of the patients, cause of death, PMI, and tPMI were analyzed. Correlations between the CRP results from various instruments were evaluated using Pearson's correlation coefficient.
Ethics statement
All cases involved medicolegal autopsies which were performed with a court-issued warrant at the request of the public prosecutor. The study was confirmed as a research activity involving non-human participants by the Institutional Review Board of Chonnam National University Hospital (No. 2019-001). All data were analyzed anonymously. The requirement for informed consent was waived by the board.
RESULTS
General characteristics
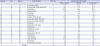
The POC tests for CRP were performed in 85 cases which included 52 males and 33 females. The mean age was 49.0 years, with a range from 1 to 92 years. The manner of death was considered natural and unnatural in 51 (60.0%) and 34 (40.0%) cases, respectively. In the POC test, CRP was elevated in 20 cases (CRP > 1.0 mg/dL) with a mean, minimum, and maximum of 5.8 mg/dL, 1.4 mg/dL, and > 20 mg/dL, respectively. The CRP levels and general characteristics, including the causes of death in cases involving elevated postmortem CRP results, are listed in
Table 1.
Table 1
Cases with positive postmortem CRP data
|
Case No. |
Sex |
Age, yr |
Case of death |
PMI, day |
Postmortem CRP, mg/dL |
|
Afinion AS100 |
BT1500 |
Cobas 8000 |
|
1 |
F |
57 |
Acute pancreatitis |
6 |
17.0 |
12.4 |
14.8 |
|
9 |
M |
18 |
Sudden adult death syndrome |
1 |
6.7 |
7.4 |
4.7 |
|
10 |
F |
59 |
Head injury |
2 |
> 20.0 |
16.8 |
29.6 |
|
14 |
F |
38 |
Panperitonitis |
2 |
4.9 |
4.3 |
3.6 |
|
18 |
F |
79 |
Drowning |
1 |
1.5 |
2.0 |
1.5 |
|
26 |
M |
61 |
Pneumonia |
3 |
5.4 |
6.7 |
3.8 |
|
36 |
M |
48 |
Sudden cardiac death |
1 |
12.0 |
13.9 |
7.8 |
|
38 |
M |
60 |
Acute myocardial infarction |
1 |
2.6 |
3.4 |
2.6 |
|
44 |
F |
79 |
Acute myocardial infarction |
2 |
2.3 |
3.1 |
2.2 |
|
49 |
M |
43 |
Drowning |
3 |
3.6 |
5.1 |
3.6 |
|
57 |
F |
41 |
Subdural hemorrhage |
2 |
4.7 |
6.9 |
4.4 |
|
61 |
F |
61 |
Intoxication |
1 |
1.4 |
1.9 |
1.6 |
|
71 |
M |
47 |
Mesentery rupture |
1 |
2.3 |
2.7 |
1.9 |
|
72 |
M |
49 |
Pneumonia |
3 |
2.8 |
3.8 |
2.5 |
|
74 |
M |
30 |
Respiratory infection |
2 |
3.2 |
4.1 |
2.7 |
|
76 |
M |
44 |
Intoxication |
4 |
3.4 |
4.2 |
3.3 |
|
78 |
M |
47 |
Liver abscess |
8 |
18.9 |
16.9 |
9.1 |
|
80 |
F |
47 |
Liver cirrhosis |
2 |
10.0 |
11.9 |
7.9 |
|
81 |
M |
43 |
Pneumonia |
2 |
4.3 |
5.1 |
3.7 |
|
83 |
F |
48 |
Subdural hemorrhage |
2 |
3.6 |
4.5 |
3.2 |
|
Average |
2.5 |
5.8 |
6.9 |
5.7 |
Comparison between antemortem and postmortem results
Antemortem CRP results were available in 12 of the 85 cases. The antemortem and postmortem CRP results were in agreement and disagreement in 9 and 3 cases, respectively (
Table 2). The PMI and tPMI in theses 12 cases were 3.7 and 4.2 days, respectively. The difference between tPMI and PMI was less than 2 days. The postmortem CRP levels were slightly lower than the corresponding antemortem levels, although there was no significant difference. The positive predictive value, specificity, negative predictive value, and sensitivity of POC CRP testing was 100%, 100%, 50%, and 66.7%, respectively.
Table 2
Comparison of antemortem CRP concentration and postmortem CRP concentration measured by a point-of-care instrument

|
Case No. |
Sex |
Age, yr |
Case of death |
PMI, day |
tPMI, day |
CRP, mg/dL |
|
Antemortem |
Postmortem |
|
2 |
M |
92 |
Abdominal stab wound |
2 |
2 |
1.3 |
< 0.5 |
|
17 |
M |
68 |
Chest injury |
3 |
3 |
0.1 |
< 0.5 |
|
20 |
F |
68 |
Hypovolemic shock |
14 |
14 |
1.2 |
0.9 |
|
26 |
M |
61 |
Pneumonia |
3 |
3 |
13.5 |
5.4 |
|
29 |
M |
44 |
Hypovolemic shock |
1 |
2 |
0.1 |
< 0.5 |
|
44 |
F |
79 |
Acute myocardial infarction |
2 |
4 |
3.1 |
2.3 |
|
61 |
F |
61 |
Intoxication |
1 |
3 |
1.9 |
1.4 |
|
68 |
M |
52 |
Retroperitoneal hemorrhage |
4 |
4 |
0.6 |
< 0.5 |
|
70 |
F |
4 |
Acute myocarditis |
2 |
2 |
1.6 |
0.6 |
|
71 |
M |
47 |
Mesentery rupture |
1 |
2 |
3.1 |
2.3 |
|
72 |
M |
49 |
Pneumonia |
3 |
3 |
5.9 |
2.8 |
|
78 |
M |
47 |
Liver abscess |
8 |
8 |
32.5 |
18.9 |
|
Average |
3.7 |
4.2 |
6.3 |
4.3 |
Comparison between POC and laboratory instruments results
Cardiac blood samples from all 85 cases were evaluated using the portable POC instrument (Afinion AS100) and the two laboratory instruments (BT1500 and Cobas 8000). Pearson's correlation coefficient analyses revealed strong correlations between the CRP levels from the portable POC instrument and the BT1500 instrument (
r = 0.971,
P < 0.001) and the Cobas 8000 instrument (
r = 0.911,
P < 0.001) (
Fig. 1).
Fig. 1
Comparison of C-reactive protein results between point-of-care test and laboratory tests. High correlations were observed between the C-reactive protein results (A) from the Afinion AS100 (point-of-care testing insrtrument) and BT1500 (r = 0.971), and (B) from the Afinion AS100 and Cobas 8000 (r = 0.911).

Error results
Error results were detected in 15 of the 85 cases, with 13 cases of errors from the POC instrument and 2 cases of errors from the laboratory instruments. Among the 13 cases of erroneous POC testing, laboratory testing revealed a valid CRP results in 9 cases and errors in 4 cases (
Table 3).
Table 3
Cases with erroneous CRP results

|
Case No. |
Sex |
Age, yr |
Case of death |
PMI, day |
Postmortem CRP, mg/dL |
|
Afinion AS100 |
BT1500 |
Cobas 8000 |
|
13 |
M |
65 |
Hypertrophic cardiomyopathy |
2 |
Error |
Error |
Error |
|
19 |
F |
54 |
Liver cirrhosis |
3 |
Error |
0.9 |
0.9 |
|
33 |
M |
44 |
Carbon monoxide intoxication |
2 |
Error |
Error |
Error |
|
34 |
M |
52 |
Carbon monoxide intoxication |
2 |
Error |
0.3 |
0.4 |
|
35 |
F |
28 |
Carbon monoxide intoxication |
2 |
Error |
0.3 |
0.3 |
|
40 |
F |
41 |
Death due to fire |
2 |
Error |
1.6 |
1.5 |
|
50 |
M |
60 |
Gastric ulcer bleeding |
3 |
Error |
< 0.01 |
< 0.03 |
|
53 |
M |
50 |
Intracerebral hemorrhage |
2 |
0.8 |
Error |
Error |
|
60 |
F |
64 |
Carbon monoxide intoxication |
3 |
Error |
< 0.01 |
< 0.03 |
|
62 |
M |
45 |
Sudden cardiac death |
3 |
< 0.5 |
Error |
Error |
|
64 |
M |
41 |
Acute myocardial infarction |
1 |
Error |
< 0.01 |
< 0.03 |
|
65 |
F |
43 |
Death due to fire |
1 |
Error |
< 0.01 |
0.3 |
|
66 |
F |
59 |
Drug intoxication |
2 |
Error |
Error |
4.0 |
|
79 |
M |
54 |
Death due to fire |
2 |
Error |
< 0.01 |
0.5 |
|
82 |
M |
77 |
Acute myocardial infarction |
2 |
Error |
Error |
Error |
DISCUSSION
According to the POC tests, CRP was elevated in 20 cases, with a mean level of 5.8 mg/dL. The putative causes for elevated CRP levels include pneumonia, liver abscess, etc. These elevated CRP results correlated with antemortem clinical data and postmortem examination results including an autopsy and histologic examination. Moreover, the present study indicated that POC CRP testing had a high positive predictive value and specificity between the antemortem and postmortem CRP results. In the 3 cases with a disagreement between antemortem and postmortem CRP results, the antemortem CRP levels were 1.2–1.6 mg/dL and the postmortem CRP results were < 1.0 mg/dL. In these cases, both POC and laboratory instruments provided negative CRP values (< 1.0 mg/dL), which indicated that these results were not performance errors of the portable POC testing instrument. Therefore, it was suggested this disagreement was due to postmortem changes. A previous study reported that postmortem CRP levels decreased by approximately 35%, compared to the antemortem CRP levels.
5
The CRP results from the portable POC instrument were highly correlated with the CRP results from the laboratory instruments (r = 0.971 and r = 0.911). However, false negative results should be considered during interpretation.
The portable POC instrument showed testing errors in 13 of the 85 cases, which were mainly related to low blood sample quality or high/low hematocrit levels. These problems are an inevitable limitation of a postmortem blood sample and are most likely caused by postmortem hemolysis and hemoconcentration. Among the 15 cases with error results, 13 cases had errors from the portable POC instrument, with detectable CRP levels from the laboratory instrument in 9 cases. Thus, failure of the POC testing should be addressed using laboratory testing of pre-treated samples. Interestingly, POC testing of whole blood samples provided testing errors in all 4 cases of carbon monoxide intoxication, which indicates that laboratory testing after sample preparation is necessary in carbon monoxide intoxication cases. Further, these error results might be due to change of hemoglobin into carboxyhemoglobin.
A previous study has described POC CRP testing in the autopsy setting using the NycoCard
®.
6 However, this study used serum samples to correct the CRP values for hematocrit levels. In contrast, the POC instrument from the present study evaluated whole blood samples, and it automatically corrected for the hematocrit levels. Thus, it is a simpler and faster method for POC testing than the instrument described in the previous study. Moreover, immediate POC CRP testing without sample preparation may facilitate decision-making regarding the autopsy procedure and may increase the frequency of usage at the death scene investigation. In this study, whole blood was collected from the IVC assuming it was right heart blood. However, in the previous report, the postmortem CRP levels were similar in the heart and peripheral blood samples.
3
Laboratory testing of postmortem blood samples is difficult due to hemolysis and postmortem blood clotting which could induce laboratory device failure by blocking the capillary. In this study, the POC testing device uses a cartridge, and thus, postmortem clotted blood does not enter into the POC testing device and does not induce a POC testing device failure. Therefore, POC testing overcomes the disadvantages of laboratory testing to some degree. We conclude that immediate POC CRP testing is reliable in forensic settings such as during autopsy and the postmortem inspection.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download