Abstract
Extramedullary myeloma refers to the presence of myeloma deposits outside the skeletal system and typically indicates a poor prognosis associated with shorter overall survival and progression- free survival. We report a case of extramedullary myeloma with extensive, abdominal multi-organ involvement mimicking lymphoma at initial diagnosis. Bulky retroperitoneal masses with severe diffusion restriction and patency of encased vessels can be MR findings of both myeloma and lymphoma. Radiologic findings such as arterial hyperenhancement, obstructive uropathy, and the lack of associated lymphadenopathy may favor a diagnosis of myeloma over lymphoma.
초록
골수외 형질 세포종은 골수 바깥에 형질세포가 침착하는 질환으로 생존기간이 짧고 예후가 불량하다. 골수외 형질 세포종이 진단 당시 광범위한 복부 장기 침범의 형태로 발현하여 림프종으로 오인되었던 증례를 보고하고자 한다. 거대한 후복막 종괴가 자기공명영상에서 강한 확산 제한을 보이고, 둘러싼 혈관의 개통이 유지되어 있는 소견은 림프종뿐만 아니라 골수종에서도 보일 수 있다. 그러나, 동맥기 조영 증강, 요로 폐색의 소견과, 동반된 림프절 종대가 없는 소견은 림프종보다는 골수종을 더 시사하는 영상소견이다.
Multiple myeloma is the malignant counterpart of long-lived plasma cells with a strong tropism for bone and bone marrow, and it is mostly confined to medullary sites (1). Extramedullary myeloma refers to the presence of myeloma deposits outside the skeletal system. Clinically or radiologically detectable extramedullary myelomas occur in approximately 10–16% of patients with multiple myeloma, and the incidence of extramedullary disease has increased in recent decades (2). However, radiologically, extramedullary myeloma is not a familiar disease. We report a case of multiple myeloma with extramedullary lesions mimicking lymphoma, with multi-organ involvement of the abdomen at the time of diagnosis.
A 58-year-old man was transferred to our hospital with a two-week history of right upper quadrant pain and weight loss. His past medical history was hypertension. Initial laboratory studies disclosed the following: hemoglobin 11.6 g/dL, hematocrit 35.1%, leukocyte count 8370/µL, platelet count 239000/µL, erythrocyte sedimentation rate 109 mm/1h, blood urea nitrogen 21.4 mg/dL, serum creatinine 1.37 mg/dL, aspartate aminotransferase 69 IU/L, alanine aminotransferase 36 IU/L, alkaline phosphatase 32 IU/L, γGT 27 IU/L, bilirubin 0.5 mg/dL, Creactive protein 18.15 mg/L, amylase 387 IU/L, lipase 580 IU/L.
Because ultrasonography (US) performed at an outside clinic showed a pancreatic head mass and gallbladder (GB) wall thickening, pancreas-biliary dynamic contrast-enhanced magnetic resonance (MR) imaging was planned. MR images demonstrated huge retroperitoneal masses involving the pancreas head and right perirenal area (Fig. 1A). A perirenal mass measuring about 15 cm appeared as an arterial hyperenhancing lesion, and showing ipsilateral mild hydronephrosis. The mass encased the inferior vena cava, and right renal vessels (Fig. 1B). The vessels showed only stretch and mild narrowing without occlusion. A large pancreatic head mass showed arterial hyperenhancement compared with the uninvolved parenchyma. The bile duct and the pancreatic duct were not significantly dilated (Fig. 1C). There were other masses in the pancreatic tail, GB, left adrenal gland, liver, and stomach. The GB lesion appeared as arterial hyperenhancing wall thickening with relatively preserved mucosal line in the fundus. The hepatic lesion was a single lesion of approximately 1.5 cm in size located in segment 4 and showed hypointensity compared to normal liver parenchyma on contrast-enhanced T1-weighted image (WI). The gastric lesion was seen as about 2 cm sized intraluminal protruding mass on the body and showed signal intensity similar to that of gastric fold on T2WI. The left adrenal lesion was a well-defined lobular mass measuring about 3.5 cm and showed signal intensity similar to that of the perirenal mass on T1- and T2WI. All masses showed significant diffusion restriction, which exceeded than spleen (Fig. 1D). And there were multiple focal lesions with restricted diffusion in the vertebrae and both ribs. Because of the multiple bone lesions, the huge and homogeneous retroperitoneal masses without vascular occlusion or ductal dilatation, and the multi-organ involvement, hematologic malignancy such as lymphoma was considered.
18F-fluorodeoxyglucose positron emission tomography-computed tomography was performed. Innumerable hypermetabolic lesions appeared in the axial skeleton. All masses in the abdomen observed on MR images were also identified as intense hypermetabolic lesions (Fig. 1E).
A percutaneous US-guided 18-gauge gun biopsy was performed on the right perirenal mass. Histologic examination was performed (Fig. 1F), and the pathologic diagnosis was plasma cell myeloma. Chemotherapy followed by autologous stem cell transplantation was planned. However, intracranial hemorrhage occurred after the initiation of chemotherapy and the patients expired after 3 weeks.
Extramedullary myeloma indicates a poor prognosis associated with decreased overall survival and decreased progression-free survival. Bladé et al. (3) proposed two mechanisms to explain the development of extramedullary myeloma. The first, involves the contiguous extraskeletal extension of myelomatous masses. The less common second mechanism involves the hematogenous dissemination of a subclone of myeloma cells that exhibit decreased expression of cell surface adhesion receptors, allowing bone marrow escape. In as many as 45% of patients with extramedullary myeloma, the tumor occurs at the time of relapse, particularly in patients treated with allogenic bone marrow transplant (23). To the best of our knowledge, there are no previous reports of extramedullary myeloma with multi-organ involvement at the time of multiple myeloma diagnosis.
In a retrospective study using MR images, the most common extramedullary lesions were paraspinal and epidural masses adjacent to the bone (39%), followed by lesions of the central nervous system head and neck (20%), abdomen (11%), thorax (8.3%), and retroperitoneum (6.9%) (4). In our patient, myelomatous masses suspected of hematogenous spread were seen in the abdomen, including the visceral organs and the retroperitoneum. It is noteworthy that extramedullary myeloma, which especially occurs apart from the bone, usually involves the visceral organs of the abdomen (45). In the abdomen, the organ most commonly involved is the liver, followed by the pancreas, stomach, and retroperitoneum (45).
In this case, bulky perirenal and pancreatic masses without ductal obstruction or vascular occlusion were misdiagnosed as lymphoma. The perirenal location is frequently involved in hematological diseases, and the high rate of disease involvement at this site is believed to be related to the activity of primitive angiohematopoietic stem cells (6). When the pancreas is involved, both lymphoma and myeloma are more common in the head because the head contains the greatest amount of native lymphoid tissue (7). In this patient, a huge perirenal mass encased the vessels. This caused only stretch and mild narrowing of the vessels without occlusion, but with accompanied mild hydronephrosis. In previous reports, large retroperitoneal myelomatous masses have demonstrated encasement of vessels without occlusion, and have occasionally caused obstructive uropathy (8). Our patient lacked biliary/pancreatic ductal dilatation or jaundice, despite the large pancreatic head mass. Unlike our patient, several reports have described bile duct dilatation or obstructive jaundice due to extramedullary myeloma involvement in the pancreas (8). Although jaundice is an infrequent finding in most patients with pancreatic lymphoma, obstructive jaundice has be reported in patients with pancreatic non-Hodgkin's disease. (9).
In diffusion-weighted MR images, lymphoma is characterized by significantly restricted diffusion, similar to the spleen (9). In our case, myeloma also showed significantly restricted diffusion. This may be explained by the fact that myeloma belongs to a lymphoid tumor. In dynamic contrast-enhanced MR images, most myelomatous masses show arterial hyperenhancement. It is known that pancreatic myeloma usually demonstrates early enhancement that is greater than that of the adjacent uninvolved pancreatic parenchyma on CT and MR imaging (9). Hypervascularity of myelomatous lesions is frequently documented (6), and this is unlike pancreatic lymphoma, which usually shows only mild enhancement.
In our case, the GB involvement of an arterial hyperenhancing mass was also demonstrated. To our knowledge, GB involvement in myeloma is extremely rare, and there have been only seven reported antemortem cases in the literature (10).
Cases of bulky retroperitoneal masses with intense restricted diffusion and patency of encased vessels usually suggest lymphoma, but multiple myeloma should be included in the differential diagnosis. Findings including arterial hyperenhancement, obstructive uropathy, and lack of associated lymphadenopathy may favor a diagnosis of myeloma over lymphoma.
Figures and Tables
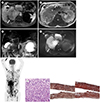
Fig. 1
A 58-year-old man with extensive extramedullary myeloma in abdomen mimicking lymphoma.
A. An arterial-phase dynamic contrast-enhanced magnetic resonance image revealing a highly enhancing right perirenal mass (arrow) with IVC encasement. The pancreatic mass (black arrow) shows hyperenhancement compared with the uninvolved pancreatic parenchyma (not shown). A hyperenhancing mass in the gallbladder fundus (arrowhead) is also shown.
B. A T2-weighted image showing masses encased in the IVC (white arrow) and two right renal arteries (arrowheads). The vessels show only mild narrowing and maintain their patency. Only mild right hydronephrosis is indicated (black arrow).
C. Magnetic resonance cholangiopancreatography image showing the cut-off of the pancreatic duct at the body (arrow). No substantial upstream ductal dilatation is observed. Furthermore, the bile duct is not dilated.
D. Masses involving the right perirenal area (arrow), pancreas (black arrow), and gallbladder (arrowhead) showing severe diffusion restriction compared to the spleen (asterix) on diffusion-weighted imaging.
IVC = inferior vena cava
E. An 18F-fluorodeoxyglucose PET-CT image showing multiple hypermetabolic skeletal lesions and a huge abdominal hypermetabolic mass.
F. A photomicrograph of tissue obtained using ultrasound-guided percutaneous gun biopsy of the perirenal mass showing a sheet of small cell infiltration. A high-power view (hematoxylin and eosin, × 400) showing abundant plasma cells containing eccentric abundant basophilic cytoplasm, eccentric round-to-oval nuclei, and fine to coarsely clumped chromatin (left). Tumor cells are positive for CD138 (a marker of plasma cell differentiation, × 40, middle) and κ light chain restriction (× 40, right) showing strong, diffuse, brown staining.
References
2. Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010; 21:325–330.

3. Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011; 29:3805–3812.
4. Tirumani SH, Shinagare AB, Jagannathan JP, Krajewski KM, Munshi NC, Ramaiya NH. MRI features of extramedullary myeloma. AJR Am J Roentgenol. 2014; 202:803–810.

5. Damaj G, Mohty M, Vey N, Dincan E, Bouabdallah R, Faucher C, et al. Features of extramedullary and extraosseous multiple myeloma: a report of 19 patients from a single center. Eur J Haematol. 2004; 73:402–406.

6. Monill J, Pernas J, Montserrat E, Pérez C, Clavero J, Martinez-Noguera A, et al. CT features of abdominal plasma cell neoplasms. Eur Radiol. 2005; 15:1705–1712.

7. Mishra MV, Keith SW, Shen X, Bar Ad V, Champ CE, Biswas T. Primary pancreatic lymphoma: a populationbased analysis using the SEER program. Am J Clin Oncol. 2013; 36:38–43.
8. Sedlic A, Chingkoe C, Lee KW, Duddalwar VA, Chang SD. Abdominal extraosseous lesions of multiple myeloma: imaging findings. Can Assoc Radiol J. 2014; 65:2–8.

9. Manning MA, Paal EE, Srivastava A, Mortele KJ. Nonepithelial neoplasms of the pancreas, part 2: malignant tumors and tumors of uncertain malignant potential from the radiologic pathology archives. Radiographics. 2018; 38:1047–1072.
10. St Romain P, Desai S, Bean S, Jiang X, Burbridge RA. Extramedullary plasmacytoma of the gallbladder diagnosed by endoscopic ultrasound fine needle aspiration (EUS-FNA). J Gastrointest Oncol. 2015; 6:E7–E9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download