Abstract
Purpose
To classify anomalous left brachiocephalic vein (LBCV) in adult without cardiac anomaly, and evaluate CT findings of anomalous LBCV.
Materials and Methods
This study included 32 patients who were diagnosed anomalous LBCV using MDCT between March 2005 and August 2016. Subaortic LBCV divided into group I (with normal LBCV) and group II (without normal LBCV). We evaluated age, sex, diameters and diameter ratios of superior vena cava (SVC) and subaortic LBCV, the entering sites to SVC of subaortic LBCV and the azygos vein, and vascular tortuosity of subaortic LBCV.
Results
There were included 29 subaortic LBCV and 3 retroesophageal LBCV. There were not statistically significant in age, sex, diameter of SVC between subaortic groups (p > 0.05). The diameters of subaortic LBCV were thinner in group I. Diameter ratios of subaortic LBCV were lower in group I. The entering site of subaortic LBCV was higher than azygos vein in group I (64%) and same as azygos vein in group II (67%). Vascular tortuosity of subaortic LBCV was in 7 cases of group I.
The normal course of left brachiocephalic vein (LBCV) is obliquely downward, passing anterior to the aortic arch and its major branches. LBCV rarely takes anomalous course. It was first described by Kershner as LBCV with subaortic position in 1888 (1). The wide application of noninvasive imaging techniques such as sonography, CT and MRI has increased incidence of anomalous LBCV (23). Most of anomalous LBCV course below the aortic arch and behind the ascending aorta. This pattern of anomalous LBCV is called subaortic or retroaortic LBCV (4). The classification of anomalous brachiocephalic vein has been suggested several authors. Takada et al. (5) supposed four major patterns of anomalous brachiocephalic vein, which is the most cited classification (5). Chern et al. (6) reported ten anomalous brachiocephalic vein subpatterns in right and left aortic arch groups. It has been reported a few cases of double LBCV, retroesophageal LBCV and retrotracheal LBCV (78), which were not included in the earlier classification suggested by Chen et al. and Takada et al. (45). Most of the articles for anomalous LBCV were obtained in patients with congenital heart disorders and relatively small numbers.
This study is to classify anomalous LBCV in adult without cardiac anomaly, and evaluate CT findings of anomalous LBCV.
This retrospective study was approved by the Institutional Review Board of our hospital (IRB No. SCHCA 2019-08-025). The requirement for informed patient consent was waived.
This study included 32 patients who were diagnosed anomalous LBCV using multi-detector computed tomography (MDCT) between March 2005 and August 2016 at a single tertiary center in our hospital, using medical and imaging records. The anomalous LBCV was defined as the LBCV passing abnormal course other than normal pathway (anterior and superior to aortic arch and anterior to its branches).
All the chest CTs were performed with 8-channel MDCT (GE LightSpeed Ultra; GE Medical System, Milwaukee, WI, USA), a 64-channel MDCT (LightSpeed VCT; GE Medical Systems) and a 256 channel MDCT (Brilliance iCT; Philips Medical Systems, Cleveland, OH, USA). CT images were restored as DICOM files after being reconstructed with slice thickness of 1.25 mm and an interval of 1.25 mm. Using three-dimensional image analysis program (Portal workstation V2.6.0.32, Philips Medical Systems), we acquired coronal views, maximum intensity projection, and three-dimensional volume rendering images. CT images were reviewed by two radiologists (K.Y.T and Y.H.W) with 26 and 3 years of experience in interpreting thoracic CT in consensus.
An anomalous LBCV passing beneath the aortic arch and anterior to trachea and esophagus was diagnosed the subaortic LBCV. Since the normal venous structure entering the superior vena cava (SVC) cannot be seen in subaortic area, all subaortic LBCV with/without normal LBCV were included in this study. An anomalous LBCV passing inferiorly along the aortic arch and running posterior to trachea and esophagus was diagnosed the retroesophageal LBCV. Since LBCV communicates to the azygos vein through the left superior intercostal vein and accessory hemiazygos vein in some peoples with normal LBCV, we included retroesophageal LBCV without normal LBCV in this study.
We divided patients with subaortic LBCV into two groups: group with normal LBCV (group I) and the group without normal LBCV (group II), and then compared CT findings between two groups. First, we measured diameters of subaortic LBCV and SVC and diameter ratios of subaortic LBCV to SVC. At the entering site of subaortic LBCV on axial image, the maximum anterior-posterior (AP) and transverse diameters of SVC were measured, and then mean value was obtained by half sum of the maximum AP and transverse diameters. Maximum AP diameter along the course and AP diameter at entering site of subaortic LBCV were measured. We compared age, sex, and diameters and diameter ratios between two groups. Second, the entering sites to SVC of subaortic LBCV and the azygos vein were compared in each patient. Third, we evaluated the vascular tortuosity of subaortic LBCV. In addition, aortic arch anomaly and other vascular malformation were evaluated.
We used Pearson's chi square test for age between subaortic two groups. Mann Whitney U test was used for sex, diameter of SVC, diameter and diameter ratios of subaortic LBCV and SVC between subaortic two groups. Fisher exact test was used for the entering sites of subaortic LBCV and azygos vein. Three cases of retroesophageal LBCV did not statistically evaluate. Statistical significance was considered when p value was less than 0.05.
Thirty two patients had an anomalous LBCV. Twenty nine patients (mean: 61.9 ± 2.9 years, 26–87 years; 15 females, 14 males) had subaortic LBCV, and three patients had retroesophageal LBCV (15 years, 57 years, 78 years; 3 males) (Fig. 1). Fourteen cases were in subaortic group I (Fig. 2) and fifteen cases in group II (Fig. 3). There were not statistically significant in age, sex, diameter of SVC between subaortic two groups. Diameters and diameter ratios of subaortic LBCV and SVC were statistically different between subaortic two groups. The diameters of subaortic LBCV were thinner in group I (maximum diameter: 6.11 ± 2.20 mm, diameter at the entering site: 4.94 ± 1.18 mm) than group II (maximum diameter: 15.01 ± 3.51 mm, diameter at the entering site: 9.87 ± 2.15 mm). Diameter ratios of subaortic LBCV were lower in group I. Anatomical relation of the entering sites of subaortic LBCV and azygos vein was statistically different between subaortic two groups. The entering site of subaortic LBCV was higher than azygos vein in group I (64%), and was same as azygos vein in group II (67%). Seven cases of subaortic group I showed vascular tortuosity (Fig. 4) (Table 1). There were right aortic arch in three cases (Fig. 5), double aortic arch in one case, and cervical aortic arch in one case.
Three retroesophageal LBCV passed downward aortic arch, routed posterior to trachea and esophagus and joined the azygos vein (Figs. 6, 7). Two retroesophageal LBCV passed inferiorly along the lateral wall of aortic arch (Fig. 6), and the other one passed inferiorly along the medial wall of aortic arch (Fig. 7).
Gerlis and Ho (9) estimated the incidence of anomalous LBCV to be 0.2% in necropsy series. Of patients with congenital heart disease, Choi et al. (10) reported incidence of anomalous LBCV was 0.98% using echocardiography and Chen et al. (4) reported 1.7% using CT. This difference may be due to the difference in the study population and the method of diagnosis. The incidence of subaortic brachiocephalic vein was 5% in patients with tetralogy of fallot (TOF) (10).
Several authors classified an anomalous LBCV by relation of the anomalous vein with aortic arch and the arterial duct or ligament (59). Takada et al. (5) supposed four patterns of anomalous LBCV: a, anomalous LBCV passing above aortic arch and behind its major branches; b, anomalous LBCV passing beneath the aortic arch, above the pulmonary artery, and in front of arterial duct or ligament; c, anomalous LBCV passing beneath the aortic arch, above the pulmonary artery, and behind arterial duct or ligament; d, anomalous LBCV passing below the pulmonary arterial trunk. Chern et al. (6) reported ten anomalous brachiocephalic vein subpatterns in right and left aortic arch groups, on basis of the relationship between the anomalous brachiocephalic vein and adjacent vessel anomalies. Ming et al. (7) reported four cases of retroesophageal LBCV in Chinese children using MDCT. Yigit et al. (8) reported a case of double LBCV with the anterior branch above the aortic arch and behind the brachiocephalic artery and the posterior branch coursed posterior to the trachea and esophagus. Classification including double and retroesophageal LBCVs has not been reported, to our knowledge.
In this study, there were subaortic LBCV without normal LBCV (47%), subaortic LBCV with normal LBCV (44%) and retroesophageal LBCV (9%). Fifteen cases of subaortic group II are relevant to type b or c by classification supposed by Takada et al. (5) The differentiation of type b and type c by Takada et al. (5) can difficult by CT on adult, since the ligamentum arteriosus cannot be detected on adult. Most cases of an anomalous LBCV are subaortic LBCV anterior to ligamentum arteriosus (511). Fourteen cases of subaortic group I are relevant to the most common subtype of circumaortic or double subaortic LBCV. In this study, there were not statistically significant in age, sex, diameter of SVC between subaortic two groups. Diameters and diameter ratios of subaortic LBCV and SVC were statistically different between two groups. The diameters of subaortic LBCV were thinner in group I than group II, and the diameter ratios of subaortic LBCV were lower in subaortic group I. Anatomical relation of the entering sites of subaortic LBCV and azygos vein to SVC was statistically different between two groups. The entering site of subaortic LBCV was higher in group I. Takada et al. (5) reported that most of subaortic LBCVs enter SVC at the same level or caudal to the azygos arch. Our seven cases of subaortic group I showed vascular tortuosity (Fig. 4). The tortuous subaortic LBCV has not been reported yet. Also, we couldn't find the tortuous subaortic LBCV in case figures of earlier reported articles.
The incidence of double LBCV has not been reported yet. Takada et al. (5) reported eight cases of subaortic LBCV, and two cases of them were double LBCV. One case of four retroesophageal LBCV reported by Ming et al. (7) was double LBCV with retroesophageal LBCV and a thinner supra-aortic LBCV. The numbers of patients were similar in subaortic two groups in this study, so subaortic group I was more common than the earlier articles. The visualization of thinner subaortic LBCV with normal positioned LBCV can be affected by the methods of contrast enhancement and CT scan thickness. We expect that intravenous contrast injection through left sided arm and thinner scan thickness can increase the incidence of subaortic group I (Fig. 4).
The exact mechanism of an anomalous LBCV is still unknown. Adachi has suggested double precardinal anastomoses (11): a superior or dorsal plexus and an inferior or ventral plexus. The regression of superior plexus results in subaortic LBCV. Abnormal development of the aortic arch and the pulmonary artery reduces the direct pressure on the upper transverse capillary plexus and subaortic LBCV develops. The right aortic arch and cervical aortic arch cause restriction of the preaortic space and enlargement of the subaortic space, and increase the possibility for the development of subaortic LBCV. In this study, there were right aortic arch in three cases, double aortic arch in one case, and cervical aortic arch in one case. Right aortic arch was reported in 21–86% of patients in an anomalous LBCV (26912). In the patients of TOF, right aortic arch was in 17% of patients without subaortic LBCV and 76% with subaortic LBCV (2). The doubled LBCV anomaly is thought to be a consequence of both ventral and dorsal anastomosis of precardinal veins (4).
Retroesophageal LBCV is a very rare anomaly. Ming et al. (7) reported the incidence of retroesophageal LBCV was 0.06% in total 6228 children, 0.19% in patients with congenital heart disease and none in patients without congenital heart disease. Our cases had no history of congenital heart disease. In one case of double LBCV of Yigit et al. (8), the posterior branch coursed posterior to the trachea and esophagus and they called it retrotracheal aberrant LBCV. In some articles, retroesophageal LBCV was reported as absence of LBCV with venous return through the left superior intercostal vein. As mechanisms of retroesophageal LBCV, Ming et al. (7) suggested the interruption of the upper anastomosis between right and left precardinal veins, no connection between the left precardinal vein and common cardinal vein, and the failure of development of the lower alternative anastomotic capillary plexus. The connection exists between the accessory hemiazygos vein and the left superior intercostal vein develops, and then retroesophageal LBCV develops without normal and subaortic LBCV. The incidence of persistent left SVC was significantly reduced in patients with an anomalous LBCV in comparison with those individuals with normal LBCV (4).
Anomalous LBCV is usually asymptomatic and diagnosed incidentally. However, it is important for radiologists to be familiar with CT appearance since anomalous LBCV can mimic a mediastinal lymphadenopathy (Fig. 4), persistent left SVC, a high positioned pulmonary artery and an atrophic right pulmonary artery. Also, anomalous LBCV may be confused with aortic dissection. Clinically, the anomalous LBCV can cause technical difficulties during pacemaker insertion, or central venous catheter insertion in left arm approach. For patient undergoing cardiac surgery, cannulation of SVC for a cardiopulmonary bypass should be done more caudally to avoid obstruction of the anomalous LBCV, because it enters the SVC more caudally than usual.
In occlusion or severe stenosis of LBCV or both LBCV or right brachiocephalic vein due to malignant or inflammatory processes, subaortic LBCV will become a major route for drainage of upper extremities (13).
Our study had some limitations. First, this study is retrospective study. Therefore, it is probably susceptible to selection bias. Second, the number of patient is small.
In conclusion, anomalous LBCV included 14 subaortic LBCV with normal LBCV, 15 subaortic LBCV without normal LBCV and 3 retroesophageal LBCV. In subaortic LBCV with normal LBCV, diameters of subaortic LBCV were thinner and diameter ratios of subaortic LBCV were lower than subaortic LBCV without normal LBCV. Seven cases of subaortic LBCV with normal LBCV showed vascular tortuosity. Three retroesophageal LBCV passed downward aortic arch, routed posterior to trachea and esophagus and joined the azygos vein. It is important for radiologists to be familiar with the CT appearance of anomalous LBCV, since the radiologists give information of uncommon or rare anomalous LBCV to surgeon or physician.
Figures and Tables
 | Fig. 1Types of anomalous LBCVs. Anomalous LBCV includes subaortic and retroesophageal LBCVs.Subaortic LBCV is defined as an anomalous LBCV passing beneath the aortic arch and anterior to the trachea and esophagus regardless of the presence of a normal LBCV, because the normal venous structure entering the superior vena cava is not in the subaortic area. Therefore, a subaortic LBCV is classified into two types according to the presence of a normal LBCV. Retroesophageal LBCV is defined as an anomalous LBCV passing inferiorly along the aortic arch and running posterior to the trachea and esophagus without the presence of a normal LBCV, because the LBCV communicates with the azygos vein through the left superior intercostal vein and accessory hemiazygos vein in some individuals with a normal LBCV.
Ao = aorta, LBCV = left brachiocephalic vein, PA = pulmonary artery, S = superior vena cava
|
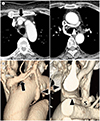 | Fig. 2Subaortic LBCV with a normal LBCV in a 64-year-old woman.
A, B. Serial axial (A) and volume-rendering (B) images show a normally positioned LBCV (arrows) and a subaortic LBCV (arrowhead; A) draining to the superior vena cava. A small subaortic LBCV has a curvilinear course (arrowheads; B). The maximum diameter and diameter of the entering site of the subaortic LBCV are 6.07 mm and 5.82 mm respectively. The mean diameter of the superior vena cava is 15.95 mm.
LBCV = left brachiocephalic vein, S = superior vena cava
|
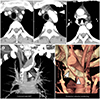 | Fig. 3Subaortic LBCV without a normal LBCV in a 43-year-old man.
A. Serial axial images show a subaortic LBCV (arrowheads) running between the ascending thoracic aorta and trachea. A normally positioned LBCV is absent anterior to the aortic arch and thoracic great vessels. The maximum diameter and diameter of the entering site of the subaortic LBCV are 15.39 mm and 7.3 mm, respectively. The mean diameter of the superior vena cava is 17.99 mm.
B. Coronal slab MIP and posterior volume-rendering images show that an subaortic LBCV (arrowheads) passes below the aortic arch and posterior to the ascending thoracic aorta, draining to the superior vena cava.
LBCV = left brachiocephalic vein, MIP = maximum intensity projection, S = superior vena cava
|
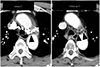 | Fig. 4Tortuous subaortic LBCV in a 74-year-old man. A tortuous subaortic LBCV is well visualized on the initial axial CT (arrowheads), but it cannot be seen on the follow up CT. This difference is caused by the injection site of the intravenous contrast media. When the initial CT was performed, intravenous contrast media was injected through the left arm. The follow up CT was performed after injection of intravenous contrast media through the right arm. On the follow up CT, the unenhanced subaortic LBCV mimics mediastinal lymph nodes.LBCV = left brachiocephalic vein
|
 | Fig. 5Subaortic LBCV with the right aortic arch in a 42-year-old man. Serial axial images show a right-sided aortic arch (arrow) and a subaortic LBCV (arrowheads) draining to the superior vena cava.LBCV = left brachiocephalic vein, S = superior vena cava
|
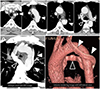 | Fig. 6Retroesophageal LBCV in a 57-year-old man.
A. Serial axial images show a subaortic LBCV (arrowheads) running lateral to the aortic arch and posterior to the descending thoracic aorta and esophagus and draining to the superior vena cava through the azygos vein (open arrowhead). A normally positioned LBCV is absent.
B. Oblique axial slab MIP and volume-rendering image with a left lateral view show a retroesophageal LBCV (arrowheads) looping around the aortic arch and descending thoracic aorta and joining the azygos vein (open arrowheads).
LBCV = left brachiocephalic vein, MIP = maximum intensity projection, S = superior vena cava
|
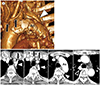 | Fig. 7Retroesophageal LBCV in a 78-year-old man.
A. Volume-rendering image with a left lateral view shows the retroesophageal LBCV (arrowhead) running medial to the Ao. Note the thyroidal veins (arrows) between the thoracic great vessels.
B. On serial axial images, an anomalous LBCV (arrowheads) passes medial to the Ao and turns posterior to the esophagus, joining the azygos vein (open arrowhead) before draining to the superior vena cava. A normally positioned LBCV is absent. Note the thyroidal veins (arrows) between the thoracic great vessels.
Ao = aortic arch, LBCV = left brachiocephalic vein, S = superior vena cava
|
References
1. Kershner L. Morphologie der vena cava inferior. Anat Anz. 1888; 3:808–823.
2. Curtil A, Tronc F, Champsaur G, Bozio A, Sassolas F, Carret JP, et al. The left retro-aortic brachiocephalic vein: morphologic data and diagnostic ultrasound in 27 cases. Surg Radiol Anat. 1999; 21:251–254.

3. Sinkovskaya E, Abuhamad A, Horton S, Chaoui R, Karl K. Fetal left brachiocephalic vein in normal and abnormal conditions. Ultrasound Obstet Gynecol. 2012; 40:542–548.

4. Chen SJ, Liu KL, Chen HY, Chiu IS, Lee WJ, Wu MH, et al. Anomalous brachiocephalic vein: CT, embryology, and clinical implications. AJR Am J Roentgenol. 2005; 184:1235–1240.

5. Takada Y, Narimatsu A, Kohno A, Kawai C, Hara H, Harasawa A, et al. Anomalous left brachiocephalic vein: CT findings. J Comput Assist Tomogr. 1992; 16:893–896.
6. Chern MS, Ko JS, Tsai A, Wu MH, Teng MM, Chang CY. Aberrant left brachiocephalic vein: CT imaging findings and embryologic correlation. Eur Radiol. 1999; 9:1835–1839.

7. Ming Z, Aimin S, Rui H. Evaluation of the anomalous retroesophageal left brachiocephalic vein in Chinese children using multidetector CT. Pediatr Radiol. 2009; 39:343–347.

8. Yigit AE, Haliloglu M, Karcaaltincaba M, Ariyurek MO. Retrotracheal aberrant left brachiocephalic vein: CT findings. Pediatr Radiol. 2008; 38:322–324.

9. Gerlis LM, Ho SY. Anomalous subaortic position of the brachiocephalic (innominate) vein: a review of published reports and report of three new cases. Br Heart J. 1989; 61:540–545.

10. Choi JY, Jung MJ, Kim YH, Noh CI, Yun YS. Anomalous subaortic position of the brachiocephalic vein (innominate vein): an echocardiographic study. Br Heart J. 1990; 64:385–387.

11. Mill MR, Wilcox BR, Detterbeck FC, Anderson RH. Anomalous course of the left brachiocephalic vein. Ann Thorac Surg. 1993; 55:600–602.

12. Adachi B. Anatomie der Japaner: II, das Venensystem der Japaner. Kyoto: Kyoto University Press;1933. p. 83–87.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download