Abstract
Purpose
To demonstrate the relationship between epicardial fat accumulation and left atrial reverse remodeling by cardiac multi-detector CT (MDCT) after catheter ablation of atrial fibrillation (AF).
Materials and Methods
Seventy-six patients underwent cardiac MDCT before and after catheter ablation of AF. Left atrial volume (LAV) and epicardial fat volume (EFV) were measured. LAV and EFV before and after catheter ablation of AF were respectively compared and the change percentages (CPs) were evaluated.
Results
The LAV after catheter ablation of AF was significantly less than the baseline LAV (107.5 ± 50.2 mL vs. 144.9 ± 62.6 mL, p < 0.001). The EFV after catheter ablation of AF was significantly greater than the baseline EFV (105.0 ± 35.6 mL vs. 90.1 ± 31.9 mL, p < 0.001). Mean CPs of LAV and EFV were −23.3% ± 20.8% and 15.9% ± 20.9%, respectively. There was a significantly negative relationship between the CPs of LAV and EFV (R = −0.53, p < 0.001).
초록
대상과 방법
76명의 환자가 심방 세동의 전극도자 절제술 전 · 후에 심장 다중 검출기 컴퓨터 단층촬영을 시행하였고, 삼차원 다중 검출기 컴퓨터단층촬영 자료를 통해 좌심방 용적과 심외막 지방의 용적이 측정되었다. 심방 세동의 전극도자 절제술 전 후의 좌심방 용적과 심외막 지방의 용적을 각각 비교하였고 그 변화를 백분율로 나타내었다
결과
심방 세동의 전극도자 절제술 이후의 좌심방 용적은 시술 이전의 기준치보다 유의하게 감소하였다(107.5 ± 50.2 mL vs. 144.9 ± 62.6 mL, p < 0.001). 또한 심외막 지방의 용적은 시술 이전의 기준치보다 유의하게 증가하였다(105.0 ± 35.6 mL vs. 90.1 ± 31.9 mL, p < 0.001). 좌심방 용적과 심외막 지방의 용적의 변화 백분율은 각각 -23.3 ± 20.8%와 15.9 ± 20.9%이었다. 좌심방 용적과 심외막 지방의 용적의 변화 백분율은 유의한 음의 상관관계를 나타내었다(R = −0.53, p < 0.001).
Atrial fibrillation (AF) is a common arrhythmia related to severe mortality and morbidity (1). In electrophysiology, AF can be viewed as a rate-related cardiomyopathy (23). Catheter ablation for electrical isolation of cardiac arrhythmogenic substrates in cardiomyopathy has gained wide acceptance for the management of drug-refractory AF (45). Since the introduction of catheter ablation, various changes of cardiac structures following catheter ablation of AF have been also reported (567).
Usually, the development and progression of AF may be associated with the electrical and structural left atrial (LA) remodeling (23). LA remodeling commonly results in the increase of LA volume (LAV) (23). Interestingly, catheter ablation of AF can make the LA chamber shrink due to ablation-induced LA wall injury (68). The shrinkage of LA chamber after catheter ablation of AF is a common finding of post catheter ablation status, and is defined as LA reverse remodeling (68).
Epicardial fat is a form of visceral fat around the heart, and acts as an important source of inflammatory mediators (91011). Epicardial fat accumulation can contribute to the development and progression of AF with the release of inflammatory mediators (1213). Generally, epicardial fat accumulation is represented by epicardial fat volume (EFV) and epicardial space thickness (EST) (131415). Previous studies demonstrated that the increase of EFV and EST may be an independent risk factor of AF recurrence after catheter ablation of AF (1316). However, to the best of our knowledge, the changes of EFV and EST after the catheter ablation of AF have not been investigated thoughtfully.
Recent cardiac CT examination using multi-detector CT (MDCT) scanner allows precise three-dimensional (3D) delineation of the LA chamber and epicardial fat surrounded by the LA wall and pericardium (1617). Thus, cardiac CT data has been widely used for the accurate measurement of LAV and EFV. In the present study, we aimed to determine the relationship between LA reverse remodeling and epicardial fat accumulation determined with MDCT in patients who underwent catheter ablation of AF.
Our Institutional Review Board approved this retrospective study, and informed consent was waived (IRB No. 2015AN0307). Based on the patient medical record system from March 2012 to March 2014, we selected 230 consecutive patients who were treated with catheter ablation of AF. Inclusion criteria were as follows: 1) adults (age > 20 years old), 2) diagnosis of drug-refractory AF, and 3) reviewable cardiac CT data before and after the catheter ablation. In our institution, patients routinely performed cardiac CT examinations before catheter ablation of AF, however this study included patients who performed cardiac CT examinations before and after catheter ablation of AF. Therefore, our study population included patients with recurrent AF, who were treated with catheter ablation of AF at least twice. To minimize confounding effects, we excluded 154 patients with 1) time interval between cardiac CT examinations of < 6 months or > 12 months (n = 80), 2) history of cardiac intervention and surgery (n = 30), 3) diabetes mellitus (n = 20), 4) hyperlipidemia (n = 15), and 5) congestive heart failure (n = 9). Eventually, this study included 76 patients who performed the cardiac CT examinations for the catheter ablation of AF at least two times with time interval of 6–12 months (Fig. 1).
Clinical data were retrospectively collected from patient medical records including age, sex, body mass index (BMI) (kg/m2) and AF burden (paroxysmal AF and persistent AF). According to the guidelines (1), paroxysmal AF was defined as recurring AF terminating in 7 days or less. Persistent AF indicated a history of AF sustaining beyond 7 days.
Electrocardiography (ECG) gated cardiac CT examinations for catheter ablation of AF were performed with a second-generation dual-source MDCT scanner (Somatom Definition Flash; Siemens Medical Solution, Erlangen, Germany). Patients with AF have irregular heartbeat rhythm characterized by temporal variations of R-R intervals (18). Therefore, retrospective ECG gating was used to minimize risk of artifacts. MDCT scanning parameters were as follows: detector collimation, 2 × 128 × 0.6 mm by means of a z-flying focal spot; gantry rotation time, 280-msec; tube voltage, 100 kV; reference tube current, 370 mAs; and pitch, 0.4. Contrast enhancement was achieved with 60 mL iopamidol (370 mg/mL iodine, Iopamiro; Bracco, Milan, Italy) injected at 5 mL/s, followed by an injection of 30 mL of diluted contrast medium (saline-to-contrast agent ratio, 7:3) and then 30 mL saline at 5 mL/s, with a power injector (Envision CT; Medrad, Indianola, PA, USA) via an antecubital vein. Cardiac CT scanning was started with the time delay determined by using a real-time bolus-tracking technique. A region of interest (ROI) was drawn in the ascending aorta to monitor an attenuation threshold of 100 Hounsfield unit (HU) above the baseline attenuation. MDCT scanning was started 6 seconds after the threshold attenuation trigger of 100 HU was reached.
Using commercially available software (Syngo; Siemens Medical Solutions), we reconstructed cardiac CT image sets in increments of 10% steps from 0% to 90% of the R-R interval on ECG. The reconstruction parameters were as follows: section thickness, 0.6-mm; increment, 0.4-mm; 470 × 470-pixel image matrix, medium smooth kernel, and 18–20-cm field of view. When all of the cardiac cycle phases were loaded, the volume rendering views of cardiac CT data sets were reformatted. The cardiac CT data set at 30% of R-R interval on ECG, which showed maximum enlargement of LA was selected for CT image analysis.
Catheter-based ablation was performed to electrically isolate the arrhythmogenic substrate related to AF. In brief, a 10F, 64-element, phased-array ultrasound catheter (AcuNav, Siemens Medical Solutions, Malvern, PA, USA) was used to visualize the interatrial septum and to guide transseptal puncture. A circular mapping catheter (Biosense Webster, Diamond Bar, CA, USA) and an ablation catheter were inserted into the LA. The 3D electroanatomic mapping of LA and PVs was performed by using EnSite NavX (Endocardial Solutions, St. Jude Medical, St. Paul, MN, USA) systems. Radiofrequency ablation was delivered with an 8-mm tipped catheter or a 3.5-mm open-irrigated tip catheter. Radiofrequency was routinely delivered for a maximum of 30 to 60 seconds to achieve a decrease in impedance of 5 to 10 U at the ablation site. And then, the LA anterior line, perimitral isthmus line, roof line, intra-coronary sinus ablation, septal line, or superior vena cava isolation were considered, if clinically needed.
Two reviewers analyzed the selected cardiac CT image set using commercial software (Terarecon iNtuition; TeraRecon, Foster City, CA, USA) independently. They were blinded to the timing of cardiac CT examination and clinical data.
To assess LA reverse remodeling, LAV was measured using 3D volumetric threshold-based method (19). The endocardial contours of LA were semi-automatically traced to select the ROI on the transverse cardiac CT slices. We applied the lowest value of CT attenuation to cover the entire contrast-enhanced LA chamber as well as to eliminate the epicardial fat within the ROI. The lowest value of CT attenuation was determined, when the LA chamber was completely included. The LA appendage and pulmonary vein confluences were excluded. Then, the LAV (in mL) was calculated from the contrast-enhanced LA chamber (Fig. 2).
To assess epicardial fat accumulation, epicardial fat was defined as the adipose tissue between the pericardium and the outer surface of heart chamber. Firstly, the contours of pericardium on transverse cardiac CT slices were semi-automatically traced between main pulmonary artery as the top boundary and the diaphragm as the lower boundary. Then, the voxels of fat density within pericardial contours were identified using threshold attenuation values of −190 to −30 HU (20). After exclusion of fat density voxel in myocardium and heart chamber, the residual voxels of fat density were defined as epicardial fat voxels. Finally, the EFV (in mL) was calculated from the epicardial fat voxels (Fig. 2).
Additionally, to assess epicardial fat accumulation around the LA chamber, EST was measured on the cardiac CT images reconstructed to 4-chamber view. In the 4-chamber view at the level of the middle LA chamber, the EST at interatrial septal groove (EST-IS) and EST at atrioventricular groove (EST-AV) were defined as the longest distance (in mm) between the apex of groove and pericardium on cardiac CT 4-chamber view, respectively (Fig. 2).
All continuous variables are expressed as mean values ± standard deviation. Interobserver and intraobserver reproducibility of MDCT measurement were assessed by using intraclass correlation coefficient. An intraclass correlation coefficient of 0.7 or greater was considered statistically reproducible (21). Comparison of MDCT measurements between before and after catheter ablation of AF was performed by using the paired Student's t-test. The change percentage (CP) of each MDCT measurement after catheter ablation of AF were calculated as the following formula: CP (in %) of MDCT measurement = (MDCT measurement after the catheter ablation-baseline MDCT measurement before the catheter ablation) / baseline MDCT measurement before the catheter ablation × 100. Furthermore, Pearson's correlation coefficient was used to determine the association between the CPs of MDCT measurements. Statistical calculations were performed by SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). p value of < 0.05 was considered statistically significant.
Baseline characteristics of 76 patients are summarized in Table 1. The mean age of 76 patients included in this study was 55.3 ± 10.2 years (range, 21 to 73 years); 67 (88.1%) of patients were male. The mean interval between cardiac CT examinations was 9.8 ± 4.3 months (range, 6 to 12 months). Radiation exposure was estimated from the dose-length product (DLP). Conversion factor of 0.014 mSv-mGy-1-cm-1 was applied for radiation dose estimation. The calculated radiation dose was 15–20 mSv (DLP range, 982–1181 mGy·cm).
Interobserver and intraobserver reproducibility of LAV, EFV, EST-IS, and EST-AV were found to be reliable with intraclass correlation coefficients of > 0.7 (Table 2). Mean baseline LAV, EFV, EST-IS, and EST-AV were 144.9 ± 62.6 mL, 90.1 ± 31.9 mL, 7.6 ± 1.8 mm, and 10.7 ± 4.5 mL, respectively. In comparison of the MDCT measurements (Table 3), LAV after catheter ablation of AF was significantly less the baseline LAV (107.5 ± 50.2 mL vs. 144.9 ± 62.6 mL, p < 0.001) (Figs. 3 and 4). EFV after catheter ablation of AF was significantly greater than the baseline EFV (105.0 ± 35.6 mL vs. 90.1 ± 31.9 mL, p < 0.001) (Figs. 3 and 4). EST-IS after catheter ablation of AF was significantly greater than the baseline EST-IS (9.5 ± 2.6 mm vs. 7.6 ± 1.8 mm, p < 0.001). EST-AV after catheter ablation of AF was also significantly greater than the baseline EST-AV (11.4 ± 4.6 mm vs. 10.7 ± 4.5 mm, p = 0.03).
In the assessment of CPs after catheter ablation of AF (Table 4), mean CPs of LAV, EFV, ESTIS, and EST-AV were −23.3 ± 20.8%, 15.9 ± 20.9%, 27.3 ± 33.7%, and 9.4 ± 31.6%, respectively. Furthermore, CPs of EFV, EST-IS, and EST-AV showed a significantly negative relationship with the CP of LAV after catheter ablation, respectively (Table 4 and Fig. 5).
This study assessed the changes of LAV, EFV, and EST determined with MDCT in patients who performed catheter ablation of AF. At 6–12 months after catheter ablation of AF, we found a significant difference of LAV, EFV, and EST before and after catheter ablation of AF. When LAV decreased in the process of LA reverse remodeling, epicardial fat also accumulated with an increase of EFV and EST after catheter ablation of AF. Furthermore, there was a significant negative relationship between LAV and epicardial fat accumulation.
Catheter ablation can isolate the substrate and trigger foci of AF with radiofrequency energy. The radiofrequency energy of catheter ablation makes LA reverse remodeling. Potential explanation of LA reverse remodeling is LA wall scarring and contraction, which may result in a remarkable decrease of LAV by 15.7% from a previous study (6). Another previous study demonstrated that there was a statistically significant relationship between medium-term procedural success and LA chamber size (22). However, remarkable reduction of LAV in the process of LA reverse remodeling after catheter ablation of AF may not guarantee better treatment outcome. This study also demonstrated that the patients who failed termination of AF after catheter ablation of AF showed a significant reduction of LAV by 23.3%. The clinical significance of epicardial fat accumulation determined with MDCT has been actively investigated in management of AF. Previous studies reported that epicardial fat accumulation determined with MDCT may act as a significant predictor for AF recurrence after catheter ablation of AF (131623). Nagashima et al. (16) reported that mean EFV was 239.0 ± 90.2 mL in patients who had recurrent AF after catheter ablation of AF. In our study results, mean baseline EFVs before catheter ablation of AF was 90.1 ± 31.9 mL in the patients who had recurrent AF. Our study showed that patients with much lower levels of EFV may have recurrent AF, compared with previous measurements of EFV after catheter ablation of AF. Although our study demonstrated the reproducibility of MDCT measurements, we were concerned by the heterogeneity of epicardial fat measurements using MDCT among various clinical institutes. It may be a critical limitation in determining the criteria to assess epicardial fat accumulation and predicting the outcome of catheter ablation of AF.
Monitoring epicardial fat accumulation may be a useful index in the management of patients with AF. Previous studies demonstrated significant change of epicardial fat during various clinical settings. Nakazato et al. (24) reported that EFV by MDCT increased with mean 13% for 4 year follow-up period in asymptomatic subjects. Furthermore, Iacobellis et al. (15) showed that epicardial fat changes are significantly associated with obesity-related cardiac morphological and functional changes during weight loss. Recent studies confirmed that epicardial fat accumulation is associated with the enlargement of LA chamber in LA remodeling (2526). In our study, EFV and EST-IS increased dramatically by mean 15.9% and 27.3% at the duration of ≤ 12 months after catheter ablation of AF. Furthermore, the increase of EFV was significantly associated with the decrease of LAV. Thus, we believe that further studies monitoring epicardial fat accumulation depending on the outcome of catheter ablation of AF may be necessary.
Our study had several limitations. First, it was a retrospective study without a normal control group. Because the study was performed retrospectively, the study group was confined to the population who had recurrent AF after catheter ablation of AF. Further studies in comparison with a control group of AF terminated with catheter ablation would help clarify the role of MDCT to determine epicardial fat accumulation. Second, other factors contributing to epicardial fat accumulation were not considered. To minimalize confounding effects, factors such as long follow-up period, history of diabetes, dyslipidemia, coronary artery disease or cardiothoracic surgery were excluded. However, other undiscovered factors could be confounding factors to our results. Third, the etiology and mechanism of increase of EFV with decrease of LAV before and after catheter ablation in patients with recurrent AF could not be determined. Further studies are required for evaluation of the etiology and mechanism of epicardial fat accumulation with LA reverse remodeling in patients with recurrent AF after catheter ablation of AF. Finally, the AF types in our study population were heterogeneous, which included persistent AF and paroxysmal AF.
In conclusion, catheter ablation of AF may result in the increase of EFV, EST-IV, and EST-AV which represents epicardial fat accumulation. Furthermore, reduction of LAV as LA reverse remodeling may be associated with epicardial fat accumulation in patients who underwent catheter ablation of AF.
Figures and Tables
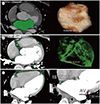 | Fig. 2Measurement of LAV, EFV, and EST using 3D cardiac CT data.
A. LAV measurement. Contrast-enhanced left atrial cavity (green) on the cardiac CT image allows total LAV measurement based on a 3D volume-rendering image of the left atrium.
B. EFV measurement. Pixels (green) of fat density (−190 to −30 Hounsfield units) within the epicardial space on cardiac CT image allow EFV measurement based on the 3D volume-rendering image of total epicardial fat.
C. EST measurement. In the measurement of EST, a four-chamber view of cardiac CT images shows the maximum distances at the EST between the myocardial surface and pericardium at interatrial groove (blue arrow) and atrioventricular groove (red arrow).
3D = three-dimensional, AV = atrioventricular groove, EFV = epicardial fat volume, EST = epicardial space thickness, IS = interatrial septal groove, LAV = left atrial volum
|
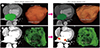 | Fig. 3Changes in LAV and EFV determined with cardiac CT images after catheter ablation of AF.
A. Left atrial chamber (green) on cardiac CT images shows a decrease in size after catheter ablation of AF. Using a 3D volume-rendering image of the left atrium from cardiac CT data, the baseline LAV was determined to be 115.0 mL. After AF ablation, LAV changed to 83.3 mL.
B. Cardiac CT images show prominent epicardial spaces (red arrows) after catheter ablation of AF. Using a 3D volume-rendering image of epicardial fat from cardiac CT data, the baseline EFV was determined to be 88.0 mL. After catheter ablation of AF, EFV changed to 132.0 mL.
3D = three-dimensional, AF = atrial fibrillation, EFV = epicardial fat volume, LAV = left atrial volume
|
 | Fig. 4Comparison of the multi-detector CT measurements obtained before and after catheter ablation of AF.
A. The lines represent the trajectory of LAV for each patient. There is a significant difference in mean LAV before and after catheter ablation of AF.
B. The lines represent the trajectory of EFV for each patient. There is a significant difference in mean EFV before and after catheter ablation of AF.
AF = atrial fibrillation, EFV = epicardial fat volume, LAV = left atrial volume
|
 | Fig. 5Relationship between the change percentages of EFV and LAV after catheter ablation of atrial fibrillation.EFV = epicardial fat volume, LAV = left atrial volume
|
References
1. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114:e257–e354.
2. Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation. 1996; 94:2968–2974.
3. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008; 1:62–73.
4. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012; 33:2719–2747.
5. Arbelo E, Brugada J, Blomström-Lundqvist C, Laroche C, Kautzner J, Pokushalov E, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur Heart J. 2017; 38:1303–1316.

6. Jayam VK, Dong J, Vasamreddy CR, Lickfett L, Kato R, Dickfeld T, et al. Atrial volume reduction following catheter ablation of atrial fibrillation and relation to reduction in pulmonary vein size: an evaluation using magnetic resonance angiography. J Interv Card Electrophysiol. 2005; 13:107–114.

7. Jeevanantham V, Ntim W, Navaneethan SD, Shah S, Johnson AC, Hall B, et al. Meta-analysis of the effect of radiofrequency catheter ablation on left atrial size, volumes and function in patients with atrial fibrillation. Am J Cardiol. 2010; 105:1317–1326.

8. Reant P, Lafitte S, Jaïs P, Serri K, Weerasooriya R, Hocini M, et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. 2005; 112:2896–2903.

9. Iacobellis G, Camarena V, Sant DW, Wang G. Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm Metab Res. 2017; 49:625–630.

10. Chang TY, Hsu CY, Chiu CC, Chou RH, Huang HL, Huang CC, et al. Association between echocardiographic epicardial fat thickness and circulating endothelial progenitor cell level in patients with stable angina pectoris. Clin Cardiol. 2017; 40:697–703.

11. Nishio S, Kusunose K, Yamada H, Hirata Y, Ise T, Yamaguchi K, et al. Echocardiographic epicardial adipose tissue thickness is associated with symptomatic coronary vasospasm during provocative testing. J Am Soc Echocardiogr. 2017; 30:1021–1027.e1.

12. Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2017; 38:1294–1302.

13. Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, et al. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010; 3:230–236.

14. Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis. 2011; 218:363–368.

15. Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring). 2008; 16:1693–1697.

16. Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, et al. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J. 2011; 75:2559–2565.

17. Stojanovska J, Cronin P, Patel S, Gross BH, Oral H, Chughtai K, et al. Reference normal absolute and indexed values from ECG-gated MDCT: left atrial volume, function, and diameter. AJR Am J Roentgenol. 2011; 197:631–637.

18. Vorre MM, Abdulla J. Diagnostic accuracy and radiation dose of CT coronary angiography in atrial fibrillation: systematic review and meta-analysis. Radiology. 2013; 267:376–386.

19. Gweon HM, Kim SJ, Kim TH, Lee SM, Hong YJ, Rim SJ. Evaluation of left atrial volumes using multidetector computed tomography: comparison with echocardiography. Korean J Radiol. 2010; 11:286–294.

20. Park HE, Choi SY, Kim HS, Kim MK, Cho SH, Oh BH. Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J Atheroscler Thromb. 2012; 19:570–576.

21. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979; 86:420–428.

22. Beukema WP, Elvan A, Sie HT, Misier AR, Wellens HJ. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation. 2005; 112:2089–2095.

23. Stojanovska J, Kazerooni EA, Sinno M, Gross BH, Watcharotone K, Patel S, et al. Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. Eur Radiol. 2015; 25:2298–2309.

24. Nakazato R, Rajani R, Cheng VY, Shmilovich H, Nakanishi R, Otaki Y, et al. Weight change modulates epicardial fat burden: a 4-year serial study with non-contrast computed tomography. Atherosclerosis. 2012; 220:139–144.

25. Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011; 57:1745–1751.

26. Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009; 119:1586–1591.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download