Abstract
Purpose
The aim of this study was to compare the measurements of diameter and volume of hepatic metastases from CT images with the overall survival and tumor response, in patients with unresectable liver metastases of colorectal cancer treated with a targeted agent.
Materials and Methods
We recruited 43 patients with unresectable liver metastases of colorectal cancer, in whom targeted therapy was used as the first-line treatment. Three-dimensional quantification of the volume of hepatic metastases was performed for each patient. An independent survival analysis using the Response Evaluation Criteria in Solid Tumors guidelines was performed and compared to the volumetric measurement. Overall survival was evaluated using the Kaplan-Meier analysis and compared to the Cox proportional hazard ratios (HRs) following univariate and multivariate analyses.
Results
In patients classified as non-progressing and progressing by the volumetric criteria, the median overall survival time was 21 months [95% confidence interval (CI): 491.25–768.75] and 11 months (95% CI: 0–949.42), respectively (p = 0.001). Using a multivariate analysis, we found that volumetric response (HR: 3.467;p = 0.002) was a significant factor affecting the overall survival in patients with liver metastases of colorectal cancer.
Go to : 
References
1. Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement.Ann Surg Oncol. 2006; 13:1271–1280.
2. Faivre J, Manfredi S, Bouvier AM. Epidemiology of colorectal cancer liver metastases.Bull Acad Natl Med. 2003; 187:815–822. ; discussion 822–823.
3. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin On- col. 2009; 27:3677–3683.

4. Alberts SR, Wagman LD. Chemotherapy for colorectal cancer liver metastases.Oncologist. 2008; 13:1063–1073.
5. Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer.N Engl J Med. 2003; 349:427–434.
6. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma.N Engl J Med. 2013; 369:722–731.
7. Trillet-Lenoir V, Freyer G, Kaemmerlen P, Fond A, Pellet O, Lombard-Bohas C, et al. Assessment of tumour response to chemotherapy for metastatic colorectal cancer: accuracy of the RECIST criteria.Br J Radiol. 2002; 75:903–908.
8. Mantatzis M, Kakolyris S, Amarantidis K, Karayiannakis A, Prassopoulos P. Treatment response classification of liver metastatic disease evaluated on imaging. Are RECIST unidimensional measurements accurate? E ur Radiol. 2009; 19:1809–1816.
9. Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size.Br J Radiol. 2000; 73:1178–1184.
10. Levine ZH, Borchardt BR, Brandenburg NJ, Clark CW, Muralikrishnan B, Shakarji CM, et al. RECIST versus.
11. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1).Eur J Cancer. 2009; 45:228–247.
12. Braga L, Guller U, Semelka RC. Pre-, peri-, and posttreatment imaging of liver lesions.Radiol Clin North Am. 2005; 43:915–927. viii.
13. Gehan EA, Tefft MC. Will there be resistance to the RECIST (Response Evaluation Criteria in Solid Tumors)? J Natl Cancer Inst. 2000; 92:179–181.

15. Prasad SR, Jhaveri KS, Saini S, Hahn PF, Halpern EF, Sumner JE. CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial.
16. Mozley PD, Schwartz LH, Bendtsen C, Zhao B, Petrick N, Buckler AJ. Change in lung tumor volume as a biomarker of treatment response: a critical review of the evidence. Ann Oncol. 2010; 21:1751–1755.

17. Nishino M, Guo M, Jackman DM, DiPiro PJ, Yap JT, Ho TK, et al. CT tumor volume measurement in advanced non-small-cell lung cancer: performance characteristics of an emerging clinical tool. Acad Radiol.
18. Shinagare AB, Jagannathan JP, Krajewski KM, Ramaiya NH. Liver metastases in the era of molecular targeted therapy: new faces of treatment response.AJR Am J Roentgenol. 2013; 201:W15–W28.
19. Krajewski KM, Braschi-Amirfarzan M, DiPiro PJ, Jagannathan JP, Shinagare AB. Molecular targeted therapy in modern oncology: imaging assessment of treatment response and toxicities.Korean J Radiol. 2017; 18:28–41.
20. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer.Ann Oncol. 2016; 27:1386–1422.
21. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018; 16:359–369.

22. Feng QY, Wei Y, Chen JW, Chang WJ, Ye LC, Zhu DX, et al. Anti-EGFR and anti-VEGF agents: important target-ed therapies of colorectal liver metastases. World J Gastroenterol. 2014; 20:4263–4275.
23. Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: review of traditional and new criteria.Rad/iiographics. 2013; 33:1323–1341.
24. Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006; 42:10311039.

25. Marten K, Auer F, Schmidt S, Kohl G, Rummeny EJ, Engelke C. Inadequacy of manual measurements compared to automated CT volumetry in assessment of treatment response of pulmonary metastases using RECIST criteria. Eur Radiol. 2006; 16:781–790.

26. Wormanns D, Kohl G, Klotz E, Marheine A, Beyer F, Heindel W, et al. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility.Eur Radiol. 2004; 14:86–92.
27. Lin M, Pellerin O, Bhagat N, Rao PP, Loffroy R, Ardon R, et al. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2012; 23:1629–1637.

28. Monsky WL, Garza AS, Kim I, Loh S, Lin TC, Li CS, et al. Treatment planning and volumetric response assessment for Yttrium-90 radioembolization: semiautomated determination of liver volume and volume of tumor necrosis in patients with hepatic malignancy. Cardiovasc Intervent Radiol. 2011; 34:306–318.

29. Reiner CS, Karlo C, Petrowsky H, Marincek B, Weishaupt D, Frauenfelder T. Preoperative liver volumetry: how does the slice thickness influence the multidetector computed tomography- and magnetic resonance-liver volume measurements?J Comput Ass/iist Tomogr. 2009; 33:390–397.
30. Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases.JAMA. 2009; 302:2338–2344.
31. Vogl TJ, Naguib NN, Eichler K, Lehnert T, Ackermann H, Mack MG. Volumetric evaluation of liver metastases after thermal ablation: longterm results following MR-guided laser-induced thermotherapy.Radiology. 2008; 249:865–871.
32. Gavrielides MA, Kinnard LM, Myers KJ, Petrick N. Noncalcified lung nodules: volumetric assessment with thoracic CT.Radiology. 2009; 251:26–37.
33. Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008; 14:1107–1115.

34. Tran LN, Brown MS, Goldin JG, Yan X, Pais RC, McNitt-Gray MF, et al. Comparison of treatment response classifications between unidimensional, bidimensional, and volumetric measurements of metastatic lung lesions on chest computed tomography. Acad Radiol. 2004; 11:1355–1360.
35. Bogaerts J, Ford R, Sargent D, Schwartz LH, Rubinstein L, Lacombe D, et al. Individual patient data analysis to assess modifications to the RECIST criteria.Eur J Cancer. 2009; 45:248–260.
36. Kim BK, Kim SU, Kim MJ, Kim KA, Kim DY, Park JY, et al. Number of target lesions for EASL and modified RECIST to predict survivals in hepatocellular carcinoma treated with chemoembolization.Clin Cancer Res. 2013; 19:1503–1511.
Go to : 
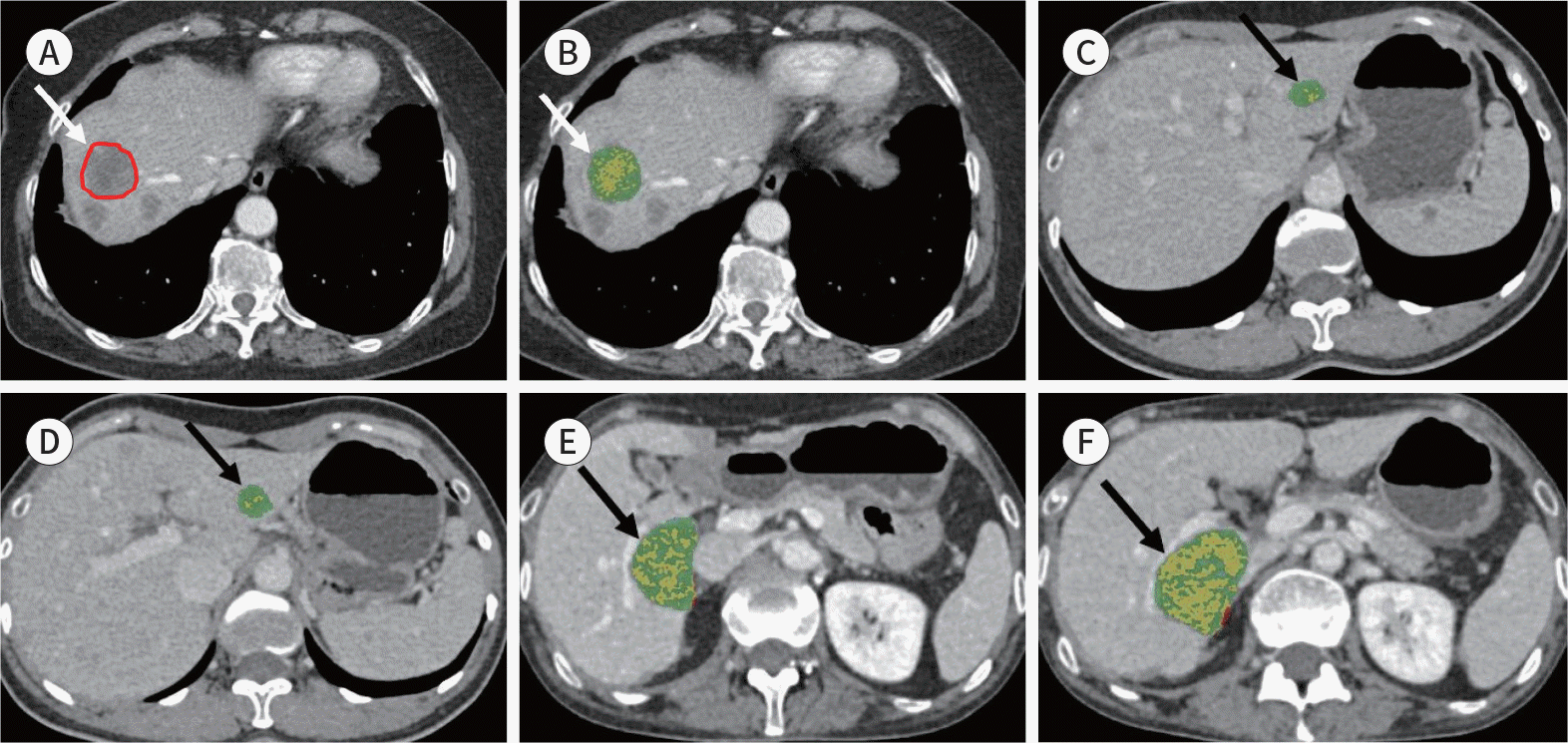 | Fig. 1.Example images of the volumetric measurement of liver metastases using the semiautomated quantification technique. A, B. For tumor margin segmentation, ROIs were manually drawn on two or three slices showing a well-delineated tumor (arrow at A). The entire tumor volume was calculated automatically (arrow at B) based on the attenuation of the drawn ROI in Hounsfield units. C, D. Example of the non-progressing group based on the volumetric criteria. CLM in a 43-year-old man is shown, with the baseline and first follow-up CT images obtained in the portal venous phase in a patient with CLM treated with bevacizumab plus FOLFIRI. At the baseline, the tumor volume was 2.7 cm3 in the left lobe of the liver (arrow at C). After the treatment, the tumor volume slightly decreased and was 2.0 cm3 (26% reduction in the total volume of the target lesion arrow at D). E, F. Example of the progressing group based on the volumetric criteria. CLM in a 47-year-old woman is shown, with the baseline and first follow-up CT images obtained in the portal venous phase in a patient with CLM treated with bevacizumab plus FOLFIRI. At the baseline, the tumor volume was 22 cm3 in the right posterior section of the liver (arrow at E). After the treatment, the tumor volume increased and was 47 cm3 (113% increase in the total volume of the target lesion; arrow at F). CLM = colorectal liver metastasis, FOLFIRI = folinic acid, fluorouracil, and irinotecan, ROI = region of interest |
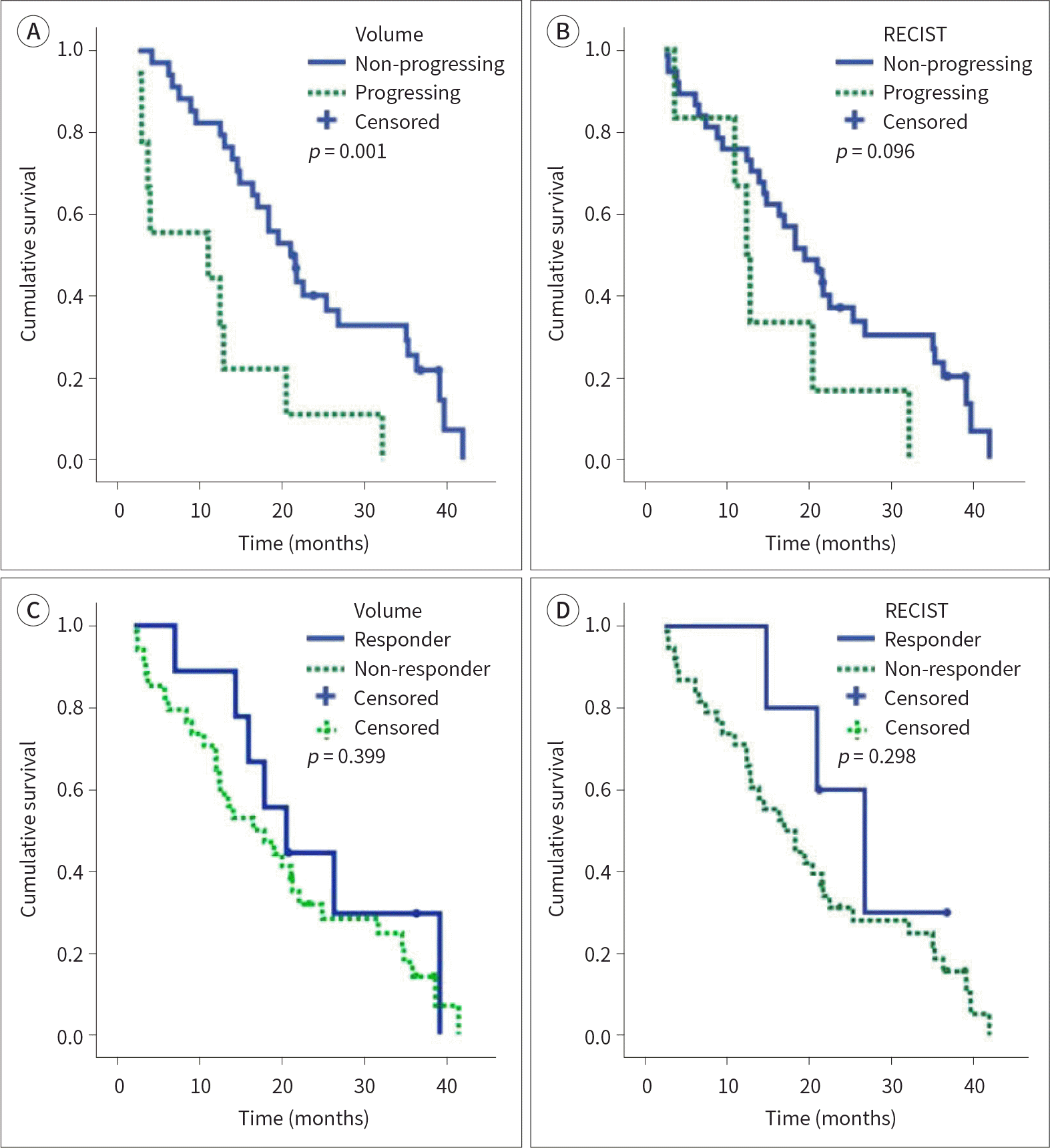 | Fig. 2.Kaplan–Meier analyses with the log-rank test to compare the overall survival in (A) the progressing and non-progressing groups based on the volumetric criteria and (B) based on the RECIST version 1.1, and in (C) the responder and non-responder groups based on the volumetric criteria and (D) based on the RE-CIST version 1.1. The median overall survival in the non-progressing group (21 months) based on the volumetric criteria was longer than that in the progressing group (11 months;p = 0.001; Log-rank test). There were no statistically significant differences in the overall survival between the progressing and non-progressing groups according to the RECIST 1.1 or between the responder and non-responder groups according to the volumetric criteria and RECIST 1.1. RECIST = Response Evaluation Criteria in Solid Tumors |
Table 1.
Baseline Patient Characteristics
Table 2.
Cross-Tabulation of RECIST and Volumetric Criteria Response to Treatment
| Volumetric Criteria | RECIST Criteria | ||||
|---|---|---|---|---|---|
| (n = 43) | CR | PR | SD | PD | Total |
| CR | 0 | 0 | 0 | 0 | 0 |
| PR | 0 | 4 | 5 | 0 | 9 |
| PR SD | 0 0 | 4 1 | 5 24 | 0 0 | 9 25 |
| PD | 0 | 0 | 3 | 6 | 9 |
| Total | 0 | 5 | 32 | 6 | 43 |
Table 3.
Prognostic Factors Affecting Survival Based on Univariate Analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download