INTRODUCTION
Thrombotic microangiopathies (TMAs) are occlusive micro/macrovascular diseases that often present with intraluminal thrombus formation. TMAs are also clinically described as microangiopathic hemolytic anemia and thrombocytopenia, for which the main forms are thrombotic thrombocytopenic purpura (TTP) and atypical hemolytic uremic syndrome (aHUS) [
12]. TTP is a hematological disorder with renal or neurological involvement and platelet-rich thrombi in small vessels [
1]. The major pathogenesis of TTP involves deficient ADAMTS13 (a disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13) activity resulting from either a genetic abnormality or the presence of inhibitory antibodies [
2]. A deficiency of ADAMTS13 activity results in the accumulation of ultra-large multimers of von Willebrand factor (VWF) due to the loss of cleavage of the 842Tyr-843Met peptide bonds in the A2 domain [
3], causing adhesion of the platelets to vascular walls. By contrast, aHUS results from hyperactivity of the alternative complement pathway due to gene mutations producing a loss of regulatory function [i.e.
CFH,
CFI,
CD46 (MCP), or
THBD] or a gain of effector function (
CFB or
C3) [
1456]. A loss of regulation that results in unrestricted complement activation leads to endothelial perturbation, leukocyte recruitment, platelet activation, tissue damage, and thrombus formation, eventually leading to end-organ failure [
7]. Although the clinical features of TTP and aHUS overlap, neurological manifestations are more prevalent in TTP, whereas aHUS typically has more severe renal involvement. Despite improvements in clinical and research-based approaches, few reliable markers that predict patient outcome have been identified.
This study examined whether complement activation products can be used as biomarkers to differentiate aHUS from TTP and whether they can predict the response to plasma exchange (PEX) treatment and the prognosis, early in the course of therapy. We focused on how complement biomarkers or ADAMTS13 activity are related to the prognosis and clinical outcomes of the patients treated for aHUS.
DISCUSSION
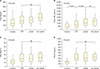
The results of this study show that complement activation via the alternative rather than the classical or lectin pathway is increased in TTP and aHUS patients. Specifically, patients showed higher levels of generalized (C3a), alternate (factor Bb), and terminal (C5a and C5b-9) markers than healthy controls, whereas the levels of C4d were not different. These findings suggest that the classical or lectin pathway complement activation does not contribute to the pathophysiology of TTP and aHUS or differentiate between the two, unlike ADAMTS13 activity. In addition, abnormal complement activation was not associated with poorer prognoses or adverse outcomes in TTP or aHUS patients.
Complement activation reflects the cooperativity among a variety of plasma proteins to fight invading microorganisms and maintain tissue homeostasis [
12]. This activation involves three pathways, namely, classical, lectin, and alternative pathways, and is regulated by soluble factors, such as factors H and I, and thrombomodulin (encoded by
THBD), and by membrane-bound regulators, including CD35, CD55, and CD59 as well as membrane cofactor protein (encoded by
CD46) [
13]. Dysregulated activation is the primary contributor to the pathogenesis of aHUS, and suppression via Eculizumab (anti-C5 monoclonal antibody) treatment is effective and safe [
4131415].
Relapse of TTP occurs in 20–30% of patients treated with PEX, and recurrence can be fatal [
16]. Therefore, the identification of novel mechanisms contributing to TTP will lead to new potentially life-saving therapeutic modalities. The observed initial complement dysregulation in TTP might exacerbate organ damage or result from microcirculation thrombi causing tissue ischemia. Ruiz-Torres et al. [
7] first reported on the complement activation in eight TMA patients. Reti et al. [
17] confirmed that levels of C3a and C5b-9 are elevated in patients with TTP compared with those in healthy controls and are significantly decreased by treatment with PEX. Subsequently, Westwood et al. [
18] demonstrated that complement activation product levels correlated with the levels of anti-ADAMTS13 IgG in TTP patients and PEX treatment significantly decreased the C3a levels. Recently Wu et al. [
19] have found that patients with elevated levels of complement activation products had a poor prognosis and the levels were elevated in both TTP patients and aHUS patients. Recent results suggest that the ultra-large vWF multimer binds to complement and is involved in the pathophysiology of the disease [
2021]. This suggests that activation of the classical pathway is not a contributor to TTP or aHUS, which is supported by our findings.
Cataland et al. [
9] suggested that complement activation biomarkers may be useful to differentiate TTP from aHUS. However, our data showed that the levels of generalized (C3a) and terminal (C5a and C5b-9) markers of complement activation were similar in both diseases, and although the median level of factor Bb was higher in the group of aHUS patients, there was a substantial overlap in levels between the patients with TTP and those with aHUS. The discrepancies between these studies may be due to the differences in the study subjects or a wide variation in the normal range of the complement activation product levels. The response rates to PEX treatment were also remarkably different (70% in our study vs. 38% reported by Cataland et al.) [
9]. Thus, the severity of the disease in the patients in the two studies may also be different. Another possible explanation is that similar levels of complement activation may be induced by sepsis or other nonspecific complications of both TTP and aHUS. However, it may be difficult to distinguish between TTP and aHUS by assessing complement activation early in the disease progression at initial presentation, whereas since the activation is sustained in aHUS patients, it is easier to distinguish the two diseases at later stages. The activation may be transient in TTP, as PEX treatment remedies the ultra-large VWF, thereby reducing complement binding [
171822]. Serial measurement during remission or after the initial treatment may be a more rational approach to evaluate the status of complement activation and to differentiate TTP from aHUS.
A severe deficiency of ADAMTS13 activity (<10%) is diagnostic for TTP, and the prolonged decrease in activity is related to poor prognosis and recurrence [
16]. Moreover, decreases in ADAMTS13 activity and associated poor prognoses have been reported for other diseases, including during severe sepsis [
23], malignant and autoimmune diseases, transplantation of solid organs [
24], and malignant hypertension [
25]. Although deficient ADAMTS13 activity does not contribute to the development of TMAs other than TTP, it has an important prognostic role. We here show that elevated ADAMTS13 activity predicts favorable clinical outcomes for patients with aHUS treated by PEX. The cause of the low ADAMTS13 activity is unknown, but perhaps, it reflects the complement-mediated binding and clearance of ADAMTS13 by VWF. Therefore, the results of our study suggest that ADAMTS13 activity can be used to determine the appropriate treatment options for aHUS patients as a result of differentiation from TTP patients, in addition to serving as a prognostic indicator to determine whether additional therapies, such as Eculizumab, should be administered.
Because of the high mortality associated with TTP, rapid diagnosis and timely treatment are crucial. Researchers at Massachusetts General Hospital recently developed the PLASMIC score as a diagnostic tool [
26]. When comparing patients with high risk (score of 6 or 7) and those with low or intermediate risk (score of ≤5), our model predicted severe ADAMTS13 deficiency with a positive predictive value of 76%, negative predictive value of 75%, sensitivity of 73%, and specificity of 78% (
Supplementary Table 3). Thus, the PLASMIC score is not sufficient to distinguish TTP from other TMAs, and ADAMTS13 activity in patients should be measured as soon as possible.
The results presented here are based on a retrospective multicenter analysis, which was limited by the relatively small number of patients. Nevertheless, TTP and aHUS are rare diseases, and obtaining larger population sizes may be challenging. Furthermore, differences in blood collection and processing among centers may result in sampling bias; however, the risk of bias is low, because these institutes have participated in a wide variety of studies with standardized blood samples. Of note, the blood samples used for the analyses in this study were collected before the start of PEX treatment. Further research involving repeated blood collection during and after the treatment is needed. Only 18% of the aHUS patients were found to have genetic mutations in our study, whereas approximately 50% of the patients in the previous studies were found to carry the genetic alterations. It seems likely that the main reason for this gap is due to the ethnic differences because other possible factors, such as Shiga-toxin–producing infection, pregnancy, chemotherapy, sepsis, or other clear causes of TMA were thoroughly excluded in our study. Given that only 21.6% of the genetic mutations were detected in the studies on pediatric aHUS patients in Korea [
27], ethnic difference is one possible explanation for this gap. Further studies with a larger cohort of patients need to be undertaken to support this possibility.
In summary, the data presented here indicate that the levels of complement activation biomarkers (except factor Bb) are similar among newly diagnosed TTP and aHUS patients. The level of ADAMTS13 activity may be useful not only for diagnosing TTP but also for predicting the clinical outcomes of the aHUS patients under PEX treatment. To the best of our knowledge, this is the first report linking high normal ADAMTS13 activity with improved outcomes of PEX treatments in aHUS patients. Given that a specific therapy exists for the treatment of aHUS, better diagnostic and prognostic tools for early recognition are necessary to improve patient outcomes These measures may distinguish patients requiring more intensive management, and the results presented here lay the groundwork for future multicenter studies aimed at identifying prognostic markers for aHUS and TMA.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download