Abstract
Background
The role of allogeneic hematopoietic cell transplantation (allo-HCT) compared with consolidation chemotherapy alone in intermediate-risk acute myeloid leukemia (AML) patients with wild-type nucleophosmin/negative or a low level of Fms related tyrosine kinase 3 internal tandem duplication (NPM1wt/FLT3-ITDneg/low) has not yet been elucidated.
Methods
In this study, we retrospectively investigated 88 patients newly diagnosed with AML who received intensive induction chemotherapy at Kyungpook National University Hospital from March 2015 to July 2017. The selection criteria included the presence of results on genetic abnormalities including NPM1 and FLT3-ITD.
Results
According to the European LeukemiaNet (ELN) risk classification, 25 patients (28%) were categorized as favorable, 44 (50%) as intermediate, and 19 (22%) as adverse risk. Among the intermediate-risk patients, 40 were identified as NPM1wt/FLT3-ITDneg/low. Among the patients with NPM1wt/FLT3-ITDneg/low, complete remission (CR) was achieved in 26 patients out of 40 (65%). One-year overall survival (OS) rate was 100% in the favorable-risk group and 87.9% in the NPM1wt/FLT3-ITDneg/low group (P=0.233). Among the intermediate-risk NPM1wt/FLT3-ITDneg/low patients, there was no survival benefit with allo-HCT (N=19) compared to consolidation chemotherapy (N=21; P=0.372). In the multivariate analysis, the ELN risk group [hazard ratio (HR), 6.36; P=0.019] and the achievement of CR (HR, 2.95; P=0.017) were both identified as factors affecting OS of patients with newly diagnosed AML.
Conclusion
Among the AML patients, intermediate-risk NPM1wt/FLT3-ITDneg/low patients and favorable-risk patients showed similar OS rates. Our results suggested that allo-HCT might have limited clinical benefit for the intermediate-risk NPM1wt/FLT3-ITDneg/low patients. Well controlled studies are needed to confirm the current results.
Acute myeloid leukemia (AML) is characterized by the clonal expansion of undifferentiated myeloid precursors, resulting in impaired hematopoiesis and bone marrow failure [12]. Previous studies have already shown the clinical and biological heterogeneity of AML [3456], and the advent of genome sequencing has revealed AML to be a highly complex and dynamic disease [78910]. Molecular and cytogenetic abnormalities are both powerful prognostic factors for AML and are included in the recent prognostic scoring system [1112131415].
Allogeneic hematopoietic cell transplantation (allo-HCT) is considered the standard treatment for most patients with AML [1617]. After completing induction chemotherapy, the decision to proceed with allo-HCT or pursue a non-transplant approach for consolidation is mainly based on risk stratification. While allo-HCT does offer the highest cure rates based on a potent graft-versus-leukemia effect, it is also associated with a considerable degree of morbidity and mortality mainly due to infections and the occurrence of graft-versus-host disease (GVHD) [1]. Therefore, the initial risk stratification assessment is becoming more important in deciding whether a patient should be a candidate for allo-HCT.
In addition to conventional cytogenetics, the results from nucleophosmin (NPM1), Fms related tyrosine kinase 3 internal tandem duplication (FLT3-ITD), and CCAAT/enhancer binding protein alpha (CEBPA) mutational screening are also now being routinely used following the 2017 European LeukemiaNet (ELN) recommendations [15]. Mutated NPM1 is known to be associated with a favorable prognosis in the absence of FLT3-ITD. However, recent studies have suggested that patients with mutated NPM1 and FLT3-ITD with a low (<0.5) allelic ratio (FLT3-ITDlow) have similar favorable outcomes as patients with mutated NPM1 only and without FLT3-ITD [181920]. Interestingly, favorable outcomes have also been reported for wild-type NPM1 and negative or low allelic ratio FLT3-ITD (NPM1wt/FLT3-ITDneg/low) patients who underwent allo-HCT, where the outcomes were similar to those of the favorable-risk group according to the ELN risk stratification [21]. Thus, while several studies have already focused on the role of allo-HCT in patients with NPM1wt/FLT3-ITDneg/low, the clinical benefits of allo-HCT compared to consolidation chemotherapy alone have not yet been elucidated [2122].
Accordingly, the present study used a retrospective analysis to evaluate the effect of allo-HCT on the long-term outcomes of newly diagnosed AML patients with NPM1wt/FLT3-ITDneg/low.
In this study, we retrospectively investigated 88 patients newly diagnosed with AML and who had received intensive induction chemotherapy at Kyungpook National University Hospital (KNUH) from March 2015 to July 2017. The other selection criteria included the presence of results regarding genetic abnormalities including NPM1 and FLT3-ITD, an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and adequate liver and renal functions for receiving intensive chemotherapy. Any patient diagnosed with acute promyelocytic leukemia, an uncontrolled infection, or uncontrolled illnesses and those treated with investigational agents or disease-modifying drugs were excluded. The disease risk stratification was determined according to the 2017 ELN recommendations [15]. This study was approved by the Institutional Review Board at KNUH.
Bone marrow and blood samples for cytogenetic and molecular analyses were obtained at the time of the initial diagnosis. The cytogenetic studies on pretreated bone marrow were performed at the time of diagnosis using unstimulated short-term (24-hour) cultures with standard G-banding. The karyotypes were interpreted using the International System for Cytogenetic Nomenclature (ISCN) criteria [23]. All molecular analyses (fluorescence in situ hybridization, chromosome banding) used in the current study applied standard methods. The screening for molecular abnormalities including NPM1 and FLT3-ITD was carried out using polymerase chain reaction (PCR) and sequencing techniques, as published earlier [2425].
All patients received a standard induction chemotherapy, which consisted of idarubicin (at a dose of 12 mg per square meter of body-surface area per day, administered by intravenous injection on days 1, 2, and 3) and cytarabine (at a dose of 200 mg per square meter, administered by continuous intravenous infusion on days 1 through 7). Those patients who achieved complete remission after induction chemotherapy received a consolidation therapy with a high dose of cytarabine (at a dose of 3.0 g per square meter, administered over a period of 3 hours every 12 hours on days 1, 3, and 5). Patients classified as high- and intermediate-risk were recommended to receive allo-HCT, while low-risk patients completed their treatment with three cycles of consolidation. The patients who underwent allo-HCT received a myeloablative conditioning (MAC, busulfan at a dose of 3.2 mg per kilogram for 4 days, plus fludarabine at 30 mg per square meter of body-surface area for 6 days) regimen or reduced intensity conditioning (RIC, busulfan at a dose of 3.2 mg per kilogram for 2 days, plus fludarabine at the same dose for 6 days) regimen according to comorbidities and the condition of the patient.
The descriptive statistics are reported as proportions and medians. Overall survival (OS) and leukemia-free survival (LFS) were calculated from the date of diagnosis to death from any cause and relapse or death from any cause, respectively. To address the time dependence of the allo-HCT, the Simon and Makuch method was used in the graphical representation and the Mantel-Byar test for the univariate analysis. Cox's regression model was used according to the method of Andersen and Gill for identifying factors for long-term survival. Factors with a P-value of less than 0.1 in the univariate analysis were then included in the multivariate analysis. The hazard ratio (HR) and 95% confidence interval (CI) were estimated for each factor. A P-value <0.05 was considered statistically significant. The statistical analyses used R statistical software 3.1.3 (the R foundation for Statistical Computing, Vienna, Austria) and SPSS for Windows (version 19.0, IBM, Chicago, IL, USA).
The median age of the 88 patients included in this study was 53 years (range, 21–69 yr), and 49 patients (56%) were men. NPM1 mutation was detected in 14 patients (16%), while 69 (79%), 9 (10%), and 10 patients (11%) exhibited negative, low, and high allelic ratio of FLT3-ITD, respectively (Supplementary Fig. 1). The ELN risk categorization identified 25 patients (28%) as favorable, 44 (50%) as intermediate, and 19 (22%) as adverse risk. Among the ELN intermediate-risk group, 40 patients were NPM1wt/FLT3-ITDlow/neg. Forty-eight patients (55%) underwent allo-HCT. The detailed patient characteristics and treatment outcomes are provided in Table 1.
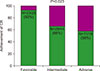
Following induction chemotherapy, 63 patients (72%) achieved a complete response (CR), whereas 25 patients (28%) experienced primary refractory disease. According to the ENL risk groups, the CR rates for the favorable, intermediate, and adverse risk groups were 92% (N=23/25), 66% (N=29/44), and 58% (N=11/19), respectively (P=0.023) (Fig. 1).
The median follow-up duration was 12.9 months (range, 1.3–39.0 mo). The 1-year OS rates were 100%, 83.5±6.9%, and 56.1±12.8% in the favorable, intermediate, and adverse risk group, respectively (P<0.001) (Fig. 2A). The 1-year leukemia-free survival (LFS) rates were 91.7±5.6%, 77.0± 6.8%, and 43.8±12.8% in the favorable, intermediate, and adverse risk group, respectively (P=0.042) (Fig. 2B). No difference in the 1-year OS rate was detected between the intermediate-risk NPM1wt/FLT3-ITDneg/low patients (87.9±5.8%) and the patients in the favorable-risk group (100%; P=0.233) (Fig. 2C). Moreover, no difference in the 1-year LFS rate was identified between the intermediate-risk NPM1wt/FLT3-ITDneg/low patients (75.6±7.7%) and the patients in the favorable-risk group (91.7±5.6%; P=0.895) (Fig. 2D).
In the univariate analysis, an adverse cytogenetic risk [hazard ratio (HR), 6.28; P=0.030], high FLT3-ITD ratio (HR, 3.52; P=0.032), CEBPA double mutation (HR, 0.24; P=0.025) the ELN risk group (P=0.008), and primary refractory disease (HR, 3.48; P=0.004) had a significant effect on OS (Supplementary Table 1). In the multivariate analysis, the ELN risk group (P=0.019) and primary refractory disease resulting from induction chemotherapy (HR, 2.95; 95% CI, 1.22–7.14; P=0.017) were identified as having a significantly adverse effect on OS (Table 2).
In the univariate analysis, a high FLT3-ITD ratio (HR, 3.20; P=0.014), the ELN risk group (P=0.016), and primary refractory disease (HR, 2.58; P=0.009) had a significant effect on LFS (Supplementary Table 2). In the multivariate analysis, the ELN risk group (P=0.030) and primary refractory disease (HR, 2.58; 95% CI, 1.27–5.22; P=0.009) were identified as having a significantly adverse effect on LFS (Table 2).
Among the 40 NPM1wt/FLT3-ITDneg/low patients in the intermediate-risk group, 19 received allo-HCT as their post-remission therapy, while the other 21 received consolidation chemotherapy alone; however, no difference was noted in the respective OS rates (Mantel-Myar test, P=0.372) (Fig. 3).
The current study evaluated the clinical outcomes for intermediate-risk AML patients with NPM1wt/FLT3-ITDneg/low and examined the therapeutic effect of allo-HCT in these patients. Comparable survival rates were noted in patients with intermediate-risk NPM1wt/FLT3-ITDneg/low and those with favorable-risk. Allo-HCT, compared to chemotherapy alone, did not show obvious superiority on long-term survival outcomes for the intermediate-risk NPM1wt/FLT3-ITDneg/low patients. The ELN risk stratification and achievement of CR were also identified as factors affecting long-term outcomes for patients with newly diagnosed AML.
While FLT3-ITD-positive patients are known to benefit significantly from allo-HCT, the benefit of allo-HCT for intermediate-risk NPM1wt/FLT3-ITDneg/low patients remains unsubstantiated [15]. In the case of cytogenetically normal AML, allo-HCT has been shown to benefit those patients without NPM mutation and without FLT3-ITD [26]. In addition, Heidrich et al. [21] have reported that allo-HCT improved OS and RFS of patients with intermediate-risk NPM1wt/FLT3-ITDneg AML compared to patients who only received post-remission consolidation chemotherapy. However, in the allo-HCT setting for normal karyotype AML, Ahn et al. [22] have reported that the triple-negative (NPM1wt/FLT3-ITDneg/non-CEBPA double mutation) group showed similar favorable long-term outcomes to the favorable-risk group according to the ELN risk classification. Moreover, the current study also found comparable survival outcomes for the intermediate-risk NPM1wt/FLT3-ITDneg/low patients and ELN favorable-risk group when using post-remission consolidation chemotherapy alone, indicating that allo-HCT may provide limited benefit to intermediate-risk NPM1wt/FLT3-ITDneg/low patients.
Furthermore, for the favorable-risk group, the current study found an improved long-term OS rate over the RFS rate, reflecting the positive role of salvage therapies, including allo-HCT (Fig. 2C, D). In contrast, the long-term OS rate for the intermediate-risk NPM1wt/FLT3-ITDneg/low group showed less improvement, indicating the limited effect of salvage therapies in this case (Fig. 2C, D). Notwithstanding, while consolidation chemotherapy seemed to be sufficient for NPM1wt/FLT3-ITDneg/low patients, the molecular heterogeneity of intermediate-risk patients should also be considered when determining the post-remission therapy. Previous studies have demonstrated that NPM1 and FLT3-ITD status provides limited prognostic information on the transplantation outcomes in cytogenetically normal AML patients [2627]. Therefore, the absence of detailed information on the baseline mutations for the present cohort and the short median follow-up duration may not be enough to reflect the long-term survival for intermediate-risk NPM1wt/FLT3-ITDneg/low patients.
The prognosis for AML depends on various patient-related factors, especially age and comorbidities, plus disease-related factors, such as chromosomal aberrations, genetic mutations, and the achievement of CR. The present study also confirmed the importance of achieving CR on patient outcomes for AML (Supplementary Table 1). While the long-term survival rates were similar for the ELN favorable-risk group and intermediate-risk NPM1wt/FLT3-ITDneg/low group, the latter showed a lower CR rate following induction therapy, where only 4 (28.6%) of 14 non-CR patients received allo-HCT (Fig. 1). Therefore, new targets and targeted agents are needed to improve the remission rates and long-term survival of intermediate-risk NPM1wt/FLT3-ITDneg/low patients. Furthermore, since consolidation chemotherapy showed similar outcomes to allo-HCT, restricting allo-HCT to patients with minimal-residual disease could be an appropriate approach for intermediate-risk NPM1wt/FLT3-ITDneg/low patients [282930].
Although the present data did not indicate a significant predictive role of allo-HCT for intermediate-risk NPM1wt/FLT3-ITDneg/low patients, the results should be cautiously interpreted due to certain limitations. First, this is a retrospective study. Second, the sample size was too small to effectively evaluate the role of allo-HCT and the factors affecting long-term outcomes for intermediate-risk NPM1wt/FLT3-ITDneg/low patients, plus the follow-up duration was relatively short and many data are censored in the chemotherapy group.
In conclusion, the current study showed similar OS rates for the intermediate-risk NPM1wt/FLT3-ITDneg/low patients and favorable-risk patients. Moreover, our results suggested that allo-HCT might have limited clinical benefits when compared with chemotherapy alone as post-remission therapy for the intermediate-risk NPM1wt/FLT3-ITDneg/low patients. Therefore, larger, well-designed prospective randomized controlled studies are needed to confirm the current results.
Figures and Tables
 | Fig. 2(A) One-year overall survival (OS) rate was 100%, 83.5±6.9%, 56.1±12.8% in favorable, intermediate, and adverse risk group, respectively. (B) One-year leukemia-free survival (LFS) rate was 91.7±5.6%, 77.0±6.8%, and 43.8±12.8% in favorable, intermediate, and adverse risk group, respectively. In patients with intermediate risk NPM1wt/FLT3-ITDneg/low, (C) one-year OS rate was 87.9±5.8%, and (D) one-year LFS rate was 75.6±7.7%, which were comparable to favorable risk group. |
 | Fig. 3Overall survival rates of NPM1wt/FLT3-ITDneg/low group according to the post-remission therapy. |
References
2. Papaemmanuil E, Dohner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med. 2016; 375:900–901.

3. Valk PJ, Verhaak RG, Beijen MA, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004; 350:1617–1628.

4. Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002; 100:4325–4336.

5. Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004; 350:1605–1616.

6. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010; 363:2424–2433.

7. Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013; 368:2059–2074.
8. Welch JS, Ley TJ, Link DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012; 150:264–278.

9. Walter MJ, Shen D, Ding L, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012; 366:1090–1098.

10. Shlush LI, Zandi S, Mitchell A, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014; 506:328–333.

11. Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012; 366:1079–1089.
12. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016; 374:2209–2221.

13. Grimwade D, Ivey A, Huntly BJ. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood. 2016; 127:29–41.

14. Meyer SC, Levine RL. Translational implications of somatic genomics in acute myeloid leukaemia. Lancet Oncol. 2014; 15:e382–e394.

15. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129:424–447.

16. Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009; 301:2349–2361.

17. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016; 127:62–70.

18. Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008; 111:2776–2784.

19. Pratcorona M, Brunet S, Nomdedeu J, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013; 121:2734–2738.

20. Schlenk RF, Kayser S, Bullinger L, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014; 124:3441–3449.

21. Heidrich K, Thiede C, Schäfer-Eckart K, et al. Allogeneic hematopoietic cell transplantation in intermediate risk acute myeloid leukemia negative for FLT3-ITD, NPM1- or biallelic CEBPA mutations. Ann Oncol. 2017; 28:2793–2798.

22. Ahn JS, Kim HJ, Kim YK, et al. Transplant outcomes of the triple-negative NPM1/FLT3-ITD/CEBPA mutation subgroup are equivalent to those of the favourable ELN risk group, but significantly better than the intermediate-I risk group after allogeneic transplant in normal-karyotype AML. Ann Hematol. 2016; 95:625–635.

23. McGowan-Jordan J, Simons A, Schmid M. ISCN 2016: an international system for human cytogenomic nomenclature (2016). Basel, Switzerland: S. Karger;2016.
24. Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002; 99:4326–4335.

25. Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood. 2006; 107:4011–4020.

26. Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008; 358:1909–1918.

27. Pasic I, Da'na W, Lam W, et al. Influence of FLT3-ITD and NPM1 status on allogeneic hematopoietic cell transplant outcomes in patients with cytogenetically normal AML. Eur J Haematol. 2019; 102:368–374.

28. Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018; 378:1189–1199.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download