Abstract
Background
Previous Caucasian studies have described venous thromboembolism in pregnancy; however, little is known about its incidence during pregnancy and early postpartum period in the Chinese population. We investigated the risk of venous thromboembolism in a “real-world” cohort of pregnant Chinese women with no prior history of venous thromboembolism.
Methods
In this observational study, 15,325 pregnancies were identified in 14,162 Chinese women at Queen Mary Hospital, Hong Kong between January 2004 and September 2016. Demographic data, obstetric information, and laboratory and imaging data were retrieved and reviewed.
Results
The mean age at pregnancy was 32.4±5.3 years, and the median age was 33 years (interquartile range, 29–36 yr). Pre-existing or newly diagnosed diabetes mellitus was present in 627 women (4.1%); 359 (0.7%) women had pre-existing or newly detected hypertension. There was a small number of women with pre-existing heart disease and/or rheumatic conditions. Most deliveries (86.0%) were normal vaginal; the remaining were Cesarean section 2,146 (14.0%). The incidence of venous thromboembolism was 0.4 per 1,000 pregnancies, of which 83.3% were deep vein thrombosis and 16.7% were pulmonary embolism. In contrast to previous studies, 66.7% of venous thrombosis occurred in the first trimester.
Conclusion
Chinese women had a substantially lower risk of venous thromboembolism during pregnancy and the postpartum period compared to that of Caucasians. The occurrence of pregnancy-related venous thromboembolism was largely confined to the early pregnancy period, probably related to the adoption of thromboprophylaxis, a lower rate of Cesarean section, and early mobilization.
Venous thromboembolism, including deep vein thrombosis and pulmonary embolism, is a major health problem. The incidence of venous thromboembolism in Western countries is approximately 1–2 cases per 1,000 persons per year [12]. The risk factors for venous thromboembolism are commonly classified as patient-related and setting-related, or reversible. Pregnancy is one of the well-recognized setting-related risk factors and pregnant women are 4–5 times more at risk compared to non-pregnant women [3]. Pulmonary embolism is a major cause of maternal mortality in the Western world. The risk factors for venous thromboembolism amongst pregnant women include increasing age (>35 yr), obesity, African-American race, inherited thrombophilia, and grand multiparity. In addition, factors that lead to immobility and bed rest such as preterm labor, premature rupture of membranes, and pre-eclampsia are the leading causes of maternal thromboembolism [4].
Contrary to the situation in Western countries, there is a paucity of literature on the epidemiology of venous thromboembolism in China. Despite this, venous thromboembolism is generally considered to be rare in our population. Nonetheless, recent major epidemiological transitions from infectious and/or nutritional deficiency diseases to lifestyle-related diseases and the modernization of the healthcare system in our population may affect the epidemiology and outcomes of pregnancy-related venous thromboembolism. This study aimed to describe the contemporary epidemiology of pregnancy-related venous thromboembolism, including the prevalence/incidence, risk factors, and outcome, in a cohort of Chinese patients in Hong Kong.
This retrospective observational study was based on a hospital-based pregnancy registry. The study protocol was approved by the local Institutional Review Board. Informed consent was not obtained from patients given the registry nature of the study; nonetheless, all patient records/information were anonymized prior to analysis. Between January 2004 and September 2016, pregnant women admitted to Queen Mary Hospital, a tertiary referral hospital affiliated with an academic institution, were identified in the computer-based clinical management system. Women were excluded if they were non-Chinese, delivered before 24-weeks' gestation and/or with a birth weight of 500 g or less, had an abortion or ectopic pregnancy, had a history of venous thromboembolism, and/or had incomplete clinical and/or follow-up data. The final analysis included a total of 15,325 pregnancies in 14,162 Chinese women. All hospital admissions, outpatient clinic visits, laboratory results, and radiological images have been recorded for all patients in the computer-based clinical management system since 1996. Demographic data, cardiovascular risk factors, and obstetric information were recorded at baseline.
Hypertension was defined as resting systolic or diastolic blood pressure ≥140/90 mmHg on two occasions or the prescription of anti-hypertensive drugs. Diabetes mellitus was defined as a serum fasting glucose ≥7.0 mmol/L or the prescription of anti-diabetic medication. Venous thromboembolism during pregnancy included 1) deep vein thrombosis and 2) pulmonary embolism during pregnancy or within 6 weeks postpartum. Only symptomatic patients with objectively confirmed lower extremity deep vein thrombosis or pulmonary embolism were included in this study. Deep vein thrombosis was objectively confirmed by ultrasonography using non-compressibility of a contiguous venous segment, as per diagnostic criteria. For pulmonary embolism, computerized tomography pulmonary angiogram or ventilation/perfusion scans were considered positive if an intraluminal filling defect was seen in a segmental or larger pulmonary artery or there was a high-probability lung scan (one or more segmental or larger perfusion defect(s) with accompanying normal ventilation seen on two different views). Patients with confirmed venous thromboembolism underwent thrombophilia screening that included the measurement of anti-cardiolipin antibodies, lupus anticoagulant, protein C and protein S, and anti-thrombin III activity. As venous thromboembolism is considered rare in Chinese, routine thromboprophylaxis such as heparin was not routinely administered. Data were retrieved from the medical records and discharge summaries of the territory-wide information network of all public hospitals in Hong Kong.
Continuous and discrete variables were expressed as means±standard deviation and percentages, respectively. Statistical comparisons of baseline clinical characteristics were performed using Student's t or Fisher's exact tests, as appropriate. The incidence of venous thromboembolism was calculated. Calculations were performed using SPSS software (version 12.0, SPSS Inc, Chicago, IL, USA). All tests were two-sided and P-values <0.05 were considered statistically significant.
From January 2004 to September 2016, a total of 15,325 pregnancies in 14,162 Chinese women were identified from the computer-based clinical management system at Queen Mary Hospital. Table 1 summarizes the clinical characteristics of the study population. The mean age at pregnancy was 32.4±5.3 years and the median age was 33 years (interquartile range, 29 and 36 yr). Of these 15,325 pregnant women, 627 (4.1%) had pre-existing or newly diagnosed diabetes mellitus, while 359 (0.7%) had pre-existing or newly detected hypertension. There was a small number of women with pre-existing valvular heart disease (0.3%) and congenital heart disease (0.5%). In addition, 27 patients (0.2%) had underlying rheumatic conditions, including 16 patients with systemic lupus erythematosus and 11 with rheumatoid arthritis. Furthermore, 19 patients (0.1%) had previous or intercurrent malignancies. Most deliveries (86.0%) were normal vaginal delivery, while 2,146 women (14.0%) underwent Cesarean section.
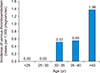
There were six cases of venous thromboembolism (five deep venous thromboses and one pulmonary embolism) during pregnancy and the post-partum period. The incidence of venous thromboembolism was 0.40 per 1,000 pregnancies, including 0.33 per 1,000 pregnancies for deep vein thrombosis and 0.007 per 1,000 pregnancies for pulmonary embolism. The age-specific incidence of pregnancy-related venous thromboembolism increased with age, from 0% per 1,000 pregnancies in those aged <30 years, to 0.51 to 0.55 per 1,000 pregnancies in those aged 30–40 years and 1.38% in those older than 40 years of age (Fig. 1). Four out of the five patients developed deep vein thrombosis (80%) during the first trimester; the remaining patient developed deep vein thrombosis in the third trimester. The only pulmonary embolism occurred in the postpartum period, four days after Cesarean section. Table 2 summarizes the clinical presentations of these six patients. Subsequent workup for thrombophilia was essentially negative for inherited thrombophilia (anti-thrombin III, protein C, and protein S activities) and antiphospholipid syndrome (lupus anticoagulant and/or anti-cardiolipin antibodies). Protein S activity was borderline low in one patient (Case IV) during pregnancy but returned to normal after delivery. There was no maternal mortality related to venous thromboembolism and no patient had any recurrent venous thromboembolism one year following cessation of oral anticoagulation therapy.
In the present study, we determined the risk of pregnancy-related venous thromboembolism in a large cohort of pregnant Chinese women. First, we showed that the overall incidence of venous thromboembolism was much lower than that in a previously reported series' from a Caucasian population. Second, the occurrence of venous thromboembolism was largely confined to the first trimester. Lastly, no hematological causes for pregnancy-related venous thromboembolism were identified.
Venous thromboembolism, including deep vein thrombosis and pulmonary embolism, remains a common vascular disease with high mortality and morbidity [5]. The risk of venous thromboembolism increases during pregnancy and the postpartum period [6]. The reported incidence of venous thromboembolism in pregnant women ranges from 0.7–1.3 per 1,000 deliveries [789], four to five times higher than that in non-pregnant women [3]. Pregnancy-related venous thromboembolism is one of the leading causes of maternal death, with a maternal mortality of 1.1–1.5 deaths per 100,000 deliveries in North America and Europe [310]. Available epidemiological data has shown that the incidence of thromboembolism in Asian populations, particularly Chinese, is considerably lower than that of Caucasian populations [9111213]. In this study, the incidence of venous thromboembolism in pregnant Chinese women in the present study was only 0.40 per 1,000 deliveries, approximately one-half to one-third that of a previous Caucasian series' as well as that of the Korean population (0.7–1.3 per 1,000 deliveries) [78913]. Similar to those previous studies, the incidence of pregnancy-related venous thromboembolism in our population appeared to increase with increasing maternal age [114] and most instances of venous thromboembolism were deep vein thrombosis [15], with all involving the left lower extremity [16], presumably related to the anatomy of the venous system draining the lower extremities. Although the reasons for the lower incidence of venous thromboembolism amongst Chinese remain unclear, it is likely to be multifactorial, with an interplay between genetic and environmental factors. For instance, the two most prevalent thrombophilic defects, factor V R506Q, also known as factor V Leiden, and prothrombin gene G20210A mutations, have frequencies of 4.4% [17] and 3.1% [18], respectively, in European populations but are extremely rare amongst Chinese [1920]. This may at least partly account for the lower overall incidence of venous thromboembolism in Chinese.
Although the risk of venous thromboembolism increases during pregnancy, the increased risk is not uniformly distributed throughout the course of pregnancy. A recent study from the United Kingdom linked primary care data from the Clinical Practice Research Datalink and secondary care from Hospital Episode Statistics between 1997 and 2010 and involved 248,953 pregnancies. The incidence of venous thromboembolism during the first trimester was only slightly higher than that during the non-pregnant period and then progressively increased, peaking around the time of delivery and the first week postpartum [21]. In stark contrast, most instances of venous thromboembolism in the present study occurred during the first trimester. Four out of the five deep vein thromboses (80%) occurred in the first trimester and one during the third trimester; the single pulmonary embolism occurred four days after Cesarean section. Consequently, the incidence of venous thromboembolism during the first trimester in the present study was comparable to or slightly lower than that of previous Caucasian series' (0.26 per 1,000 deliveries vs. 0.4–0.5 per 1,000 deliveries) [2122]. The lower overall incidence of pregnancy-related venous thromboembolism could be the result of much lower venous thromboembolism during late pregnancy and the early postpartum period.
Indeed, the predominant physiological, anatomical, and behavioral changes responsible for the occurrence of venous thromboembolism differ at different stages of pregnancy [6]. During the first trimester, well before any significant anatomical changes, pregnancy induces a hypercoagulable state, with increased synthesis of coagulation factors II, VII, VIII, and X and fibrin; reduced production of protein S; and suppressed systemic fibrinolytic activity, resulting in venous thromboembolism [2324]. With increasing gestational age, physical compression of the gravid uterus on the inferior vena cava and the pelvic veins reduces venous flow velocity and increases venous stasis in the lower extremities. This becomes increasingly important in the pathogenesis of venous thromboembolism during the later stages of pregnancy [2526]. In addition, certain antepartum events such as preterm labor, premature rupture of membranes, and pre-eclampsia necessitating bed rest and immobility are also major risk factors for maternal venous thromboembolism. Moreover, pregnant women who undergo Cesarean section have a four-fold higher risk of venous thromboembolism (2.6 per 1,000 Cesarean sections) than that in women who have a normal vaginal delivery [27]. This may be related to greater activation of coagulation in the former as well as delayed mobilization [28].
Being an affiliated hospital of an academic institution, we are a tertiary hospital accepting referrals of high-risk pregnant women. Since 2004, our institution has adopted the Green-top guidelines for thromboembolic risk stratification [29], although we employ compression stockings as a mainstay of thromboprophylaxis in most Chinese women with an intermediate risk of thromboembolism and heparin only in women with high thromboembolic risk, thrombophilia, prior venous thromboembolism, or ≥3 thromboembolic risk factors. Furthermore, our institution adopts vaginal delivery as the first-line delivery method in all patients unless contraindicated and encourages mobilization until the time of delivery and early after Cesarean section. In contrast to the high proportion of Cesarean section in Europe and North America, which typically comprises one-third of all deliveries [30], only 14% of pregnant women in the present study underwent Cesarean section. In a previous study from Hong Kong almost a decade ago, in which 20% of the women underwent Cesarean section and mobilization 36–48 hours after Cesarean section, the reported incidence of pregnancy-related venous thromboembolism was comparable to that of a previous Caucasian series of 1.88 per 1,000 deliveries [11]. In that study, 81% of venous thromboembolisms occurred in the third trimester (6%) and postpartum period (75%) and 65% of those late venous thromboembolisms occurred in patients who underwent Cesarean section. Nonetheless, only 9.4% of venous thromboembolism occurred in the first trimester (0.18 per 1,000 deliveries), which is similar to the findings of the present study. A subsequent thrombophilia study revealed only a minority of patients (−10%) with documented thrombophilia such as protein C and/or protein S deficiency [20]. The use of thromboprophylaxis, a smaller proportion of patients who underwent Cesarean section, and early mobilization after Cesarean section in the present study may underlie the difference in the incidence of venous thromboembolism during late pregnancy and the postpartum period.
Our study had several limitations. First, it was limited by its registry-based and single-center observational design. Second, Hong Kong is unique in terms of its early urbanization and Westernization. As a result, our findings cannot be generalized to less well-developed cities or suburbs in China. Third, biomarkers such as D-dimer, which are useful for the screening and/or diagnosis of venous thromboembolism when there is clinical uncertainty, were not routinely measured in our patients in a standardized manner. Therefore, our cohort represented patients with signs and symptoms of venous thromboembolism but not clinically ambiguous cases. Fourth, although thrombophilia screening was performed in all pregnant women with venous thromboembolism, genotyping of the factor V Leiden and prothrombin genes was not performed. Lastly, although we carefully ascertained all cases of venous thromboembolisms by careful examination of hospital records, laboratory and imaging results, patients with a milder form of deep vein thrombosis and/or pulmonary embolism who were not hospitalized were not included.
Our cohort of Chinese women had a substantially lower risk of venous thromboembolism during pregnancy and postpartum period than their Caucasian counterparts. The occurrence of venous thromboembolism in late pregnancy and postpartum period is rare, which could be related to the use of thromboprophylaxis, a low rate of Cesarean section, and early mobilization in our center.
Figures and Tables
References
1. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998; 158:585–593.

3. Liu S, Rouleau J, Joseph KS, et al. Epidemiology of pregnancyassociated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can. 2009; 31:611–620.

4. Okoroh EM, Azonobi IC, Grosse SD, Grant AM, Atrash HK, James AH. Prevention of venous thromboembolism in pregnancy: a review of guidelines, 2000-2011. J Womens Health (Larchmt). 2012; 21:611–615.

5. Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007; 167:935–943.

6. Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008; 6:632–637.

7. Kevane B, Donnelly J, D'Alton M, Cooley S, Preston RJ, Ni Ainle F. Risk factors for pregnancy-associated venous thromboembolism: a review. J Perinat Med. 2014; 42:417–425.

8. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005; 143:697–706.

9. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006; 194:1311–1315.

10. Mander R, Smith GD. Saving Mothers' Lives (formerly Why Mothers die): reviewing maternal deaths to make motherhood safer 2003-2005. Midwifery. 2008; 24:8–12.

11. Chan LY, Tam WH, Lau TK. Venous thromboembolism in pregnant Chinese women. Obstet Gynecol. 2001; 98:471–475.

12. Liao S, Woulfe T, Hyder S, Merriman E, Simpson D, Chunilal S. Incidence of venous thromboembolism in different ethnic groups: a regional direct comparison study. J Thromb Haemost. 2014; 12:214–219.

13. Jang MJ, Bang SM, Oh D. Incidence of pregnancy-associated venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011; 9:2519–2521.

14. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000; 160:809–815.

16. Chan WS, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ. 2010; 182:657–660.

17. Gregg JP, Yamane AJ, Grody WW. Prevalence of the factor V-Leiden mutation in four distinct American ethnic populations. Am J Med Genet. 1997; 73:334–336.

18. Mazoyer E, Ripoll L, Gueguen R, et al. Prevalence of factor V Leiden and prothrombin G20210A mutation in a large French population selected for nonthrombotic history: geographical and age distribution. Blood Coagul Fibrinolysis. 2009; 20:503–510.

20. Tam WH, Ng MH, Yiu AK, Lau KM, Cheng GY, Li CY. Thrombophilia among Chinese women with venous thromboembolism during pregnancy. Gynecol Obstet Invest. 2012; 73:183–188.

21. Abdul Sultan A, Tata LJ, Grainge MJ, West J. The incidence of first venous thromboembolism in and around pregnancy using linked primary and secondary care data: a population based cohort study from England and comparative meta-analysis. PLoS One. 2013; 8:e70310.

22. Virkus RA, Lokkegaard EC, Lidegaard O, et al. Venous thromboembolism in pregnancy and the puerperal period: a study of 1210 events. Acta Obstet Gynecol Scand. 2013; 92:1135–1142.

24. James AH. Prevention and management of venous thromboembolism in pregnancy. Am J Med. 2007; 120:S26–S34.

25. Simcox LE, Ormesher L, Tower C, Greer IA. Pulmonary thrombo-embolism in pregnancy: diagnosis and management. Breathe (Sheff). 2015; 11:282–289.

26. Macklon NS, Greer IA, Bowman AW. An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol. 1997; 104:191–197.

27. Blondon M, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of venous thromboembolism after cesarean sections: a meta-analysis. Chest. 2016; 150:572–596.

28. Epiney M, Boehlen F, Boulvain M, et al. D-dimer levels during delivery and the postpartum. J Thromb Haemost. 2005; 3:268–271.

29. SNelson-Piercy C, MacCallum P, Mackillop L. Reducing the risk of venous thromboembolism during pregnancy and the puerperium: RCOG Green-top Guideline No. 37a. London, UK: Royal College of Obstetricians and Gynaecologists;2015. Accessed December 5, 2018. at https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf.
30. Martin JA, Hamilton BE, Osterman MJ. Births in the United States, 2013. NCHS Data Brief. 2014; 1–8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download