Abstract
Drug resistance in cancer, especially in leukemia, creates a dilemma in treatment planning. Consequently, studies related to the mechanisms underlying drug resistance, the molecular pathways involved in this phenomenon, and alternate therapies have attracted the attention of researchers. Among a variety of therapeutic modalities, mesenchymal stem cells (MSCs) are of special interest due to their potential clinical use. Therapies involving MSCs are showing increasing promise in cancer treatment and anticancer drug screening applications; however, results have been inconclusive, possibly due to the heterogeneity of MSC populations. Most recently, the effect of MSCs on different types of cancer, such as hematologic malignancies, their mechanisms, sources of MSCs, and its advantages and disadvantages have been discussed. There are many proposed mechanisms describing the effects of MSCs in hematologic malignancies; however, the most commonly-accepted mechanism is that MSCs induce tumor cell cycle arrest. This review explains the anti-tumorigenic effects of MSCs through the suppression of tumor cell proliferation in hematological malignancies, especially in acute myeloid leukemia.
Acute myeloid leukemia (AML) is a type of blood malignancy which involves cells that differentiate into white blood cells. AML starts in the bone marrow (BM). It comprises a heterogeneous group of disorders characterized by the rapid expansion of immature myeloid cells (blasts) in the BM. The inability of current therapies to eradicate blasts and chemotherapy refractoriness are the major causes underlying AML progression/relapse. The high rate of mortality due to AML reinforces the need for a greater understanding of the leukemic BM microenvironment and alternative methods for treating this disorder. Stem cell therapy is one of the best candidates to treat such hematological malignancies. There are different types of stem cells, including embryonic stem cells (ESCs), fetal stem cells, mesenchymal stem cells (MSCs), and hematopoietic stem cells (HSCs) that continuously replenish certain tissues. Among the different types of stem cells, MSCs have been specifically considered for clinical applications. Since the discovery of MSCs, various studies were performed to understand their physiology, function, and behavior [1]. Therefore, it is generally reported that MSCs have multi-lineage potential and are capable of differentiating into various types of cells [2]. The multi-lineage capacity of MSCs makes them promising therapeutic targets and one of the most indispensable sources of cell therapy and regenerative medicine resources. Despite comprehensive research performed over the past 10 years, it is still unknown whether MSCs have tumor-promoting or tumor-suppressing effects. Research is required before MSCs can be used for the treatment of hematologic malignancies. In this review, we give an overview of studies regarding the use of MSCs in the treatment of AML as an example of an evolving model of myeloid malignancies (Table 1).
The pioneering work of Caplan in 1991 introduced the terms stroma and MSCs to the scientific community and indicated that MSCs are able to differentiate into the adipocyte cell lineage [3]. MSCs, as well as other types of adult stem cells, are characterized by self-renewal capability, clonogenic efficiency, and multi-lineage differentiation capacity. In general, MSCs are isolated by their capacity to adhere to culture-dish plastic surfaces. Cells can be expanded in culture plates and immunologically characterized by a specific panel of markers. Because of the lack of unique and definitive cellular markers, the characterization of MSCs remains difficult. For this reason, the International Society for Cellular Therapy suggested three minimal criteria for the characterization of MSCs: (a) plastic adherence, (b) expression of markers related to mesenchymal cells such as CD73, CD90, and CD105, and lack of hematopoietic-related cells such as CD34, CD45, CD11b or CD14, CD19 or CD79α, and HLA-DR expression, and (c) their tri-lineage differentiation potential into adipocytes, osteoblasts, and chondrocytes (Fig. 1) [4]. MSCs, which are present in adult tissues and organs such as adipose tissue, liver, kidney, heart, placenta, amniotic fluid, amnion, and BM, among others, are undifferentiated cells that have the capacity to differentiate into a broad range of different cell types, including adipocytes, neuron-like cells, osteocytes, chondrocytes, and other connective tissues [56789]. Earlier, it was demonstrated that MSCs can only differentiate into mature cells of the same organ, but recent findings have shown that these cells can also differentiate into other cell types, and even into the cells making up endoderm, mesoderm, and ectoderm [10]. Also, due to their plasticity, self-renewal, and relatively non-immunogenic properties, MSCs are potentially considered for regeneration, transplantation, and treatment of certain diseases such as ischemia, multiple sclerosis, cartilage and bone pathologies, cardiac events, auto-immune disorders, cancer, genetic diseases, and blood malignancies [1112]. Of the forementioned diseases, hematological abnormality and blood malignancy have gained more attention for cell transplantation with MSCs (Fig. 2) [13]. Some studies indicated that MSCs could release multiple angiogenic growth factors and cytokines/chemokines. These observations suggest that MSCs may have potential as a useful cell source for therapeutic angiogenesis [14]. Following these findings, Fernandez-Garcia et al. (2015) reported that adipose tissue derived-MSCs (ADSCs) improve the homing of donor HSCs and progenitor cells into recipient BM, facilitating the stable reconstitution of transplanted recipients with infused hematopoietic grafts [15]. These results open new perspectives for the application of ADSCs in HSCs therapy. Furthermore, the therapeutic potential of MSCs in veterinary medicine has been demonstrated since 2003 and MSC-based therapies have used in more than 500 dogs and 2,500 horses [16]. However, there are concerns regarding these cells and the risks linked to their therapeutic use are still unclear, particularly in the context of patients affected by pre-existing cancer [17]. It was reported that interactions between cancer cells and MSCs are of fundamental importance in stimulating both the development and invasiveness of tumors. As mentioned above, MSCs have specific features that make them candidates for cell therapy. One of the known roles of these cells was indicated by their use for the treatment of a hematological disorder [17].
Cancer cells involve a set of abnormalities, including uncontrolled cell growth, cell invasion, genetic instability, and, finally, tumor development and metastasis. Therefore, a variety of promising new therapies for cancers, such as immunomodulation and cell therapy, are being developed. Ongoing studies propose that MSCs are good targets for cell therapy in a variety of cancers. The effects of MSCs on cancer cells are yet to be controversial [13]. Some studies indicated inhibitory effects, while others reported proliferative activity. For instance, in an in vitro study, it was shown that MSCs have tumoricidal effects on breast and lung cancer cell lines [18]. Furthermore, the co-culture of MSCs and melanoma cancer cell line cells revealed the promotion of cell proliferation [19]. It was also shown that MSCs cause tumor growth when injected into mice with prostate cancer [20]. It seems that MSCs, through signaling pathways, can suppress both proliferation and apoptosis of cancer cells [2122]. This dual role of MSCs can be described as a “double-edged sword”. Therefore, understanding MSCs' dual roles in tumor cell proliferation and apoptosis is required. Identifying the function of MSCs in hematologic malignancies such as lymphoma and leukemia may be applicable to hematologic cancer treatment. Regarding some studies involving hematologic malignancies, it has been shown that MSCs are capable of promoting or inhibiting tumor growth by suppressing apoptosis, or proliferation of tumor cells, respectively (Fig. 3) [1131823]. Although few investigations have reported that MSCs can directly promote the proliferation or apoptosis of malignant cells, the predominant hypothesis is that MSCs suppress both proliferation and apoptosis [2425]. Thus, the efficacy of the use of MSCs in treatment of hematologic malignancies is poorly understood, and the mechanisms underlying the pro-tumorigenic and anti-tumorigenic effects of these cells act are currently unclear [26]. Some studies suggest mechanisms that have inhibitory effects on hematologic malignancies. In general, the mechanisms mentioned include the possible use of MSCs as a delivery vehicle [27], inhibition of vascular growth [28], or to arrest the cell cycle [2629].
Various studies indicated that MSCs can interfere hematologic malignancies via inhibiting the proliferation of tumor cells. Various sources of MSCs have been utilized for this purpose. These sources include BM, which was the first source of MSCs discovered for clinical applications; umbilical cord blood; and adipose tissue [30]. MSCs derived from these sources are known to have similar surface antigen expression phenotypes, and immunosuppressive properties [31]. Our experimental results showed that the antitumor effects of MSCs are not dependent on their tissue source and origin. Besides the cell source used, the number of MSCs and cancer cells seeded for co-culture is another important consideration. In other words, culture conditions, especially the concentration of MSCs, are known to significantly affect proliferation rate, morphology and secreted factors [32]. Moreover, it has been reported that antitumor effects in solid tumors are observed to associate with a higher number of MSCs [23]. This dependency is yet to be suggested for hematologic malignancies. There are many proposed mechanisms describing the effects of MSCs on cancer cells; however, the most commonly-accepted mechanism is that MSCs induce tumor cell cycle arrest. Most studies related to effect of MSCs on AML cells were carried out using U937, HL-60, and HL-60/VCR cell lines instead of primary cells. In this regard, Liang et al. (2008) reported that direct contact of U937, HL-60, and HL-60/VCR AML cells with human BM fibroblast stromal cells (HFCLs) causes inhibition of cell proliferation and induction of apoptosis. In their study, it was shown that upon co-culture with HFCLs, the percentage of AML cells in the G1 phase was higher and that of AML cells in the S phase cells was lower than those without HFCL cell-coculture [33]. In other words, Liang et al. (2008) suggested cell cycle G0/G1 blockage by transcriptional activation of specific genes [33]. In parallel, MSCs were found to inhibit the self-renewal ability of tumor cells. In this regard, Tian et al. (2010) demonstrated that umbilical cord (UC)-MSCs cause proliferative inhibition of HL-60 cells due to G0/G1 arrest. In addition, in this study, p38 mitogen- activated protein kinase (MAPK) was suggested as a potent suppressor of cell proliferation and tumorigenesis in this cell line [34]. In another investigation, Li et al. (2018) showed that UC-MSCs inhibited the proliferation of HL-60 and THP-1 cells as AML cell lines. Their results indicate varying effects of UC-MSCs on various types of AML cell lines associated with secreted cytokines and the expression of cytokine receptors on the cells [35]. They suggested different mechanisms, such as secretion of certain substances or paracrine signals, for the antitumor effects of UC-MSCs, besides cell cycle arrest, but the exact mechanism was not determined in their study.
As promising delivery vehicles, MSCs can be used for cancer cell therapy (Fig. 4) [3637]. These cells are easily accessible, quickly cultured in vitro, and transplanted [38]. Moreover, MSCs are known to possess hypo-immunogenic characteristics and can migrate to tumor sites [39]. It has been reported that the cytotoxic effects of MSCs may be helpful if they could identify tumor sites and migrate to it [40]. However, there have been a number of challenges in adapting the homing ability of MSCs for targeted delivery [41]. Furthermore, MSCs can also be used as gene therapy carriers [42]. In the same way, several studies have used MSCs to deliver genes and other factors, such as IL-12 [43], IL-24 [44], and IFN-γ [45] to tumor sites. Moreover, at the cellular level, MSCs exert most effects through extracellular vesicles (EVs), including microvesicles and exosomes [46]. These EVs are lipid membrane-bound vesicles secreted from MSCs. EVs contain a variety of molecules such as microRNAs, RNA, and proteins that have originated in MSCs, and these contents can be transferred to other cells, such as cancerous cells [47]. In one study by Hendijani et al. (2015), it was reported that MSC EVs produced an anti-proliferative effect on leukemic cells, and a cytotoxic effect in combination with doxorubicin, demonstrating an anti-leukemic potential of exosome-derived MSCs [48].
In recent studies, it was shown that tumor growth resulted from proangiogenic characteristics of MSCs. However, it has been documented that MSCs can impair vessel growth or angiogenesis under certain conditions. They can migrate to endothelial cell-derived-capillaries to produce reactive oxygen species (ROS) [49]. Following generation of ROS, apoptosis of endothelial cells and suppression of tumor growth take place [28]. The mechanisms involved relate to modulation of the vascular endothelial cadherin/β-catenin signaling pathway [50]. Moreover, previous studies reported that MSCs present in high numbers are potentially cytotoxic, and injection of MSCs into tumor sites may be an effective antiangiogenic treatment [49]. However, the inhibitory effect of MSCs on tumor growth has not been clearly indicated in hematologic cancers, but it may be important as these cancers are dependent on vascular support.
Cell cycle arrest is the most common fundamental process of tumor growth inhibition. Although cell cycle checkpoints and DNA repair processes seem to be linked to various cancers, mechanisms inducing cancer cell-cycle arrest by antitumor agents are yet to be identified. In other words, the effects of MSCs on leukemia, lymphoma and another blood malignancies are not well understood [51]. Some studies have shown high levels of cells arrested at G0/G1. In detail, Fonseka et al. (2012) indicated that UC-MSCs could inhibit the proliferation of K562 cells due to arrest in G0/G1 phase through IL-6 and IL-8 cytokine secretion [52]. In another study, Fathi et al. (2019) reported that BM-derived MSCs were attributed to a robust increase in the number of cells in G0/G1 phase, which implies cell arrest at G0/G1. This result agrees with earlier reports by other authors [5253]. Therefore, further research is needed to understand the mechanisms of tumor cell cycle arrest that consequently lead to the antitumor effects of MSCs in hematologic malignancies.
As mentioned above, MSCs exhibit immunoregulatory properties that influence both innate and adaptive immune responses [5455]. Moreover, it seems that MSCs inhibit erythropoiesis to favor myeloid differentiation via production of cytokines and growth factors, such as interleukin (IL)-6, which was shown to expand myeloid progenitors and block erythroid development [56]. In this context, elevated IL-6 levels have been correlated with adverse survival in patients with AML [57]. Another player engaged in the BM microenvironment (niche) regulation is the autonomic nervous system that accompanies marrow blood vessels through adrenergic fibers [58]. Deregulation of the interaction between adrenergic fibers and the MSC niche has been implicated in impaired hematopoiesis, which is a hallmark of several hematologic diseases [58]. As previously reported, as an evolving model of myeloid malignancies, AML-derived MSCs display enhanced supportive capacity for hematopoiesis by changing expression of cell surface molecules or CD markers, including CD44, CD49e, CD271 and CXCL12 [5960]. As confirmed for myelodysplastic syndrome, in AML, MSC-derived endothelial cell numbers are predominantly increased, especially in cases with rapidly proliferating disease, further suggesting MSC-derived cell implication in leukemic niche building. Moreover, it has been indicated that AML blasts can modulate endothelial cell expansion via the upregulation of E-selectin adhesion molecules and may then adhere to the stroma and be concealed in a silent status, eventually becoming chemo-resistant [61]. Recent evidence, reviewed by Huang et al. (2015), report that AML-derived MSCs from AML patients show similar CD90, CD73, CD44, and E-cadherin expression, but decreased monocyte chemoattractant protein-1 levels compared to MSCs from healthy donors [62]. Also, AML blast interactions with MSCs show that both cells release several cytokines and chemokines and, when co-cultured, normal MSCs had an anti-apoptotic and growth-enhancing effect on primary human AML cells, this was associated with upregulation of the mTOR signaling pathway [63]. Recently, it has been shown that different clinical/cytogenetic AML subgroups may show differences in MSC niches. In one study, Lopes et al. (2017) characterized and arrayed MSC cytokine expression in patients with de novo AML and secondary AML [AML with myelodysplasia related changes (MRC)]. They found that de novo AML-derived MSCs presented VEGFA, CXCL12, RPGE2, IDO, IL-1β, IL-6, and IL-32 at high levels and IL-10 in lower levels. However, AML-MRC-derived MSCs presented IL-6 at high levels [64].
Immune dysregulation of leukemic niches is an attractive approach for cellular therapies. Recently, an increasing number of reports have supported the use of immune checkpoint blockers as well as monoclonal antibody therapies engaging specific T cells in hematologic malignancies. Immune checkpoints are one of the protective mechanisms that are induced in activated T cells and which regulate T cell antigen responses. In other words, cancers can evade immune-mediated destruction by upregulation of certain molecules on the surface of T cells. Indeed, immune checkpoint blockers could enhance cytotoxicity of cytokine-induced killer cells against myeloid leukemic blasts [65]. Recently it was shown that vaccination with MSCs promotes apoptosis of tumor cells and inhibits proliferation by increasing MHC1 and heat shock protein (HSP) expression levels. In detail, the enhanced antitumor response of MSCs was strongly associated with higher expression levels of MHC class I molecules on dendritic cells (DCs) that made tumor cells more cross-presentable to host DCs to generate antitumor activity [66]. Another attractive perspective includes the optional transfer of gene-modified MSCs which secrete tumor-directed antibodies continuously into the body of the patient. As MSCs have less immunogenicity and tend to condense in the close neighborhood of the tumor, they can be used as a means for the targeted delivery of anticancer agents. Aliperta et al. (2015) reported that gene-modified MSCs are able to express a CD33-CD3 bispecific antibody and to interfere with efficient lysis of AML blasts by human T cells in AML patients [66]. With regard to antibody-derived agents, such as bispecific agents and antibody-drug conjugates, CD33 is a clinically validated target and was shown to be effective in AML treatment [66]. In addition, antibodies specific for CD123 are under evaluation [67]. Li et al. (2018) indicated that the anti-CD44 antibody A3D8 inhibits proliferation of HL-60 cells, a representative acute leukemia cell line [35]. The A3D8 treatment increased the percentage of cells in G0/G1 cell cycle phase [68]. However, other in vitro investigations reported that MSCs may escape this targeted therapy and that leukemic stem cells become less microenvironment-dependent in advanced-stage AML, so that targeting of CD44 may be less successful than expected. Other attractive therapeutic approach for myeloid disorders involve the use of allogeneic BM transplantation, chimeric antigen receptor T (CART) cells, and donor lymphocyte infusion (DLI) [69]. These approaches are presently aimed at targeting leukemic blasts, but the use of MSCs might be novel targets in the near future.
MSC-based therapeutic approaches have shown a wide range of outcomes, probably due to non-standardized experimental methods, heterogeneous characteristics of MSCs, and a lack of specific cell surface markers that are easily affected by the surrounding environment. The tumor-related effects of MSCs are still not well understood. Therefore, much more researches are necessary to develop MSCs as a cell-based therapy for cancer. Various studies have been carried out to investigate the effects of MSCs in tumorigenesis, but a single principle cannot explain the dual anti-tumorigenic and pro-tumorigenic roles of MSCs. It has been indicated that the antitumor effects of MSCs are principally a result of the suppressed proliferation of malignant cells via an arrest in the G0/G1 phase of the cell cycle [23]. In order to exploit this anti-tumorigenic feature of MSCs for clinical use in the future, more investigation is recommended.
Figures and Tables
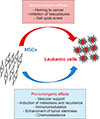 | Fig. 3Schema for the dual roles of MSCs in hematologic malignancy. MSCs have both anti-tumorigenic and pro-tumorigenic effects, as they tend to not only inhibit tumor growth but also promote tumor growth by suppressing tumor cell apoptosis. |
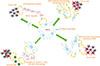 | Fig. 4MSC and tumor cell interactions as MSC-based cancer therapy. The chemotactic movement of MSCs toward a tumor niche is driven by soluble factors such as EGF, IL-6, IL-8, TGF-β, and PDGF. Genetic modification of MSCs can be used to deliver a range of tumor-suppressing cargos directly into the tumor site. These cargos include growth factors and cytokines, immune-modulating agents (IFN-α, IFN-β, IL-2, IL-12, CX3CL1 etc.), and regulators of gene expression (miRNAs and other non-coding RNAs). MSCs are also capable of delivering therapeutic drugs within the tumor site. Also, micro vesicles derived from MSCs represent an alternative approach to delivering these agents. |
Notes
References
1. Lee MW, Ryu S, Kim DS, et al. Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges. Leukemia. 2019; 33:597–611.

2. Fathi E, Farahzadi R. Isolation, culturing, characterization and aging of adipose tissue-derived mesenchymal stem cells: a brief overview. Braz Arch Biol Technol. 2016; 59:e16150383.

4. Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007; 16:91–104.

5. Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, et al. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013; 3:433–437.
6. Perrot P, Rousseau J, Bouffaut AL, et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One. 2010; 5:e10999.

7. Fathi E, Farahzadi R. Zinc sulphate mediates the stimulation of cell proliferation of rat adipose tissue-derived mesenchymal stem cells under high intensity of EMF exposure. Biol Trace Elem Res. 2018; 184:529–535.

8. Fathi E, Farahzadi R, Sheikhzadeh N. Immunophenotypic characterization, multi-lineage differentiation and aging of zebrafish heart and liver tissue-derived mesenchymal stem cells as a novel approach in stem cell-based therapy. Tissue Cell. 2019; 57:15–21.

9. Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Anti-aging protective effect of L-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell. 2018; 54:105–113.

10. Ren Y, Wu H, Zhou X, et al. Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci. 2012; 93:404–411.

11. Valenti MT, Mori A, Malerba G, Dalle Carbonare L. Mesenchymal stem cells: A new diagnostic tool? World J Stem Cells. 2015; 7:789–792.

12. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010; 5:e10088.

14. Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011; 9:10.

15. Fernandez-Garcia M, Yanez RM, Sanchez-Dominguez R, et al. Mesenchymal stromal cells enhance the engraftment of hematopoietic stem cells in an autologous mouse transplantation model. Stem Cell Res Ther. 2015; 6:165.

16. Hall MN, Rosenkrantz WS, Hong JH, Griffin CE, Mendelsohn CM. Evaluation of the potential use of adipose-derived mesenchymal stromal cells in the treatment of canine atopic dermatitis: a pilot study. Vet Ther. 2010; 11:E1–E14.
17. Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011; 17:93–106.

18. Tian LL, Yue W, Zhu F, Li S, Li W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 2011; 226:1860–1867.

19. Suzuki K, Sun R, Origuchi M, et al. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011; 17:579–587.

20. Lin G, Yang R, Banie L, et al. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010; 70:1066–1073.

21. Wu YL, Li HY, Zhao XP, et al. Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017; 108:897–909.

22. Yulyana Y, Ho IA, Sia KC, et al. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol Ther. 2015; 23:746–756.

23. Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F 3rd. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011; 29:11–19.

24. Zhu N, Wang H, Wei J, et al. NR2F2 regulates bone marrow-derived mesenchymal stem cell-promoted proliferation of Reh cells. Mol Med Rep. 2016; 14:1351–1356.

25. Lin HD, Fong CY, Biswas A, Choolani M, Bongso A. Human umbilical cord Wharton's jelly stem cell conditioned medium induces tumoricidal effects on lymphoma cells through hydrogen peroxide mediation. J Cell Biochem. 2016; 117:2045–2055.

26. Song N, Gao L, Qiu H, et al. Mouse bone marrow-derived mesenchymal stem cells inhibit leukemia/lymphoma cell proliferation in vitro and in a mouse model of allogeneic bone marrow transplant. Int J Mol Med. 2015; 36:139–149.

27. Duchi S, Sotgiu G, Lucarelli E, et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: effective photoinduced in vitro killing of osteosarcoma. J Control Release. 2013; 168:225–237.

28. Lee JK, Park SR, Jung BK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013; 8:e84256.

29. Sarmadi VH, Tong CK, Vidyadaran S, Abdullah M, Seow HF, Ramasamy R. Mesenchymal stem cells inhibit proliferation of lymphoid origin haematopoietic tumour cells by inducing cell cycle arrest. Med J Malaysia. 2010; 65:209–214.
30. Fathi E, Farahzadi R, Valipour B, Sanaat Z. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS One. 2019; 14:e0215678.

31. Russell KA, Chow NH, Dukoff D, et al. Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS One. 2016; 11:e0167442.

32. Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008; 36:1176–1185.

33. Liang R, Huang GS, Wang Z, et al. Effects of human bone marrow stromal cell line (HFCL) on the proliferation, differentiation and apoptosis of acute myeloid leukemia cell lines U937, HL-60 and HL-60/VCR. Int J Hematol. 2008; 87:152–166.

34. Tian K, Yang S, Ren Q, et al. p38 MAPK contributes to the growth inhibition of leukemic tumor cells mediated by human umbilical cord mesenchymal stem cells. Cell Physiol Biochem. 2010; 26:799–808.

35. Li Q, Pang Y, Liu T, et al. Effects of human umbilical cord-derived mesenchymal stem cells on hematologic malignancies. Oncol Lett. 2018; 15:6982–6990.

36. Peng L, Jiang D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS One. 2018; 13:e0205918.

37. Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV. Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Front Pharmacol. 2018; 9:259.

38. Garikipati VNS, Singh SP, Mohanram Y, Gupta AK, Kapoor D, Nityanand S. Isolation and characterization of mesenchymal stem cells from human fetus heart. PLoS One. 2018; 13:e0192244.

39. Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012; 7:e47559.

40. Gao Z, Zhang L, Hu J, Sun Y. Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine. 2013; 9:174–184.

41. Ramdasi S, Sarang S, Viswanathan C. Potential of mesenchymal stem cell based application in cancer. Int J Hematol Oncol Stem Cell Res. 2015; 9:95–103.
42. Dembinski JL, Wilson SM, Spaeth EL, et al. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy. 2013; 15:20–32.

43. Zhao WH, Cheng JX, Shi PF, Huang JY. Human umbilical cord mesenchymal stem cells with adenovirus-mediated interleukin 12 gene transduction inhibits the growth of ovarian carcinoma cells both in vitro and in vivo. Nan Fang Yi Ke Da Xue Xue Bao. 2011; 31:903–907.
44. Zhang X, Zhang L, Xu W, et al. Experimental therapy for lung cancer: umbilical cord-derived mesenchymal stem cell-mediated interleukin-24 delivery. Curr Cancer Drug Targets. 2013; 13:92–102.

45. Yang X, Du J, Xu X, Xu C, Song W. IFN-γ-secreting-mesenchymal stem cells exert an antitumor effect in vivo via the TRAIL pathway. J Immunol Res. 2014; 2014:318098.
46. Laso-Garcia F, Ramos-Cejudo J, Carrillo-Salinas FJ, et al. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS One. 2018; 13:e0202590.

47. Rajendran RL, Gangadaran P, Bak SS, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017; 7:15560.

48. Hendijani F, Javanmard SH, Sadeghi-aliabadi H. Human Wharton's jelly mesenchymal stem cell secretome display antiproliferative effect on leukemia cell line and produce additive cytotoxic effect in combination with doxorubicin. Tissue Cell. 2015; 47:229–234.

49. Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009; 113:4197–4205.

50. Menge T, Gerber M, Wataha K, et al. Human mesenchymal stem cells inhibit endothelial proliferation and angiogenesis via cell-cell contact through modulation of the VE-Cadherin/β-catenin signaling pathway. Stem Cells Dev. 2013; 22:148–157.

52. Fonseka M, Ramasamy R, Tan BC, Seow HF. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC) inhibit the proliferation of K562 (human erythromyeloblastoid leukaemic cell line). Cell Biol Int. 2012; 36:793–801.

53. Zhang HM, Zhang LS. Influence of human bone marrow mesenchymal stem cells on proliferation of chronic myeloid leukemia cells. Ai Zheng. 2009; 28:29–32.
54. Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015; 6:560.

55. Wang Z, Tang X, Xu W, et al. The different immunoregulatory functions on dendritic cells between mesenchymal stem cells derived from bone marrow of patients with low-risk or high-risk myelodysplastic syndromes. PLoS One. 2013; 8:e57470.

56. Iancu-Rubin C, Mosoyan G, Wang J, Kraus T, Sung V, Hoffman R. Stromal cell-mediated inhibition of erythropoiesis can be attenuated by Sotatercept (ACE-011), an activin receptor type II ligand trap. Exp Hematol. 2013; 41:155–166.e17.

57. del Toro R, Mendez-Ferrer S. Autonomic regulation of hematopoiesis and cancer. Haematologica. 2013; 98:1663–1666.

58. Arranz L, Sanchez-Aguilera A, Martin-Perez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014; 512:78–81.

59. Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009; 23:664–672.

60. Abe-Suzuki S, Kurata M, Abe S, et al. CXCL12+ stromal cells as bone marrow niche for CD34+ hematopoietic cells and their association with disease progression in myelodysplastic syndromes. Lab Invest. 2014; 94:1212–1223.

61. Pezeshkian B, Donnelly C, Tamburo K, Geddes T, Madlambayan GJ. Leukemia mediated endothelial cell activation modulates leukemia cell susceptibility to chemotherapy through a positive feedback loop mechanism. PLoS One. 2013; 8:e60823.

62. Huang JC, Basu SK, Zhao X, et al. Mesenchymal stromal cells derived from acute myeloid leukemia bone marrow exhibit aberrant cytogenetics and cytokine elaboration. Blood Cancer J. 2015; 5:e302.

63. Brenner AK, Nepstad I, Bruserud O. Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front Immunol. 2017; 8:106.

64. Lopes MR, Pereira JK, de Melo Campos P, et al. De novo AML exhibits greater microenvironment dysregulation compared to AML with myelodysplasia-related changes. Sci Rep. 2017; 7:40707.

65. Tian T, Yu S, Liu L, et al. The profile of T helper subsets in bone marrow microenvironment is distinct for different stages of acute myeloid leukemia patients and chemotherapy partly ameliorates these variations. PLoS One. 2015; 10:e0131761.

66. Aliperta R, Cartellieri M, Feldmann A, et al. Bispecific antibody releasing-mesenchymal stromal cell machinery for retargeting T cells towards acute myeloid leukemia blasts. Blood Cancer J. 2015; 5:e348.

67. Fracchiolla NS, Fattizzo B, Cortelezzi A. Mesenchymal stem cells in myeloid malignancies: a focus on immune escaping and therapeutic implications. Stem Cells Int. 2017; 2017:6720594.

68. Chen P, Huang H, Wu J, et al. Bone marrow stromal cells protect acute myeloid leukemia cells from anti-CD44 therapy partly through regulating PI3K/Akt-p27(Kip1) axis. Mol Carcinog. 2015; 54:1678–1685.

69. Bao H, Wu D. Current status of leukemia cytotherapy - exploitation with immune cells. Curr Stem Cell Res Ther. 2017; 12:188–196.

70. Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992; 79:2370–2377.

71. Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5). Exp Hematol. 2001; 29:448–457.

72. Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007; 21:304–310.

73. Zhu Y, Sun Z, Han Q, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009; 23:925–933.

74. Wei Z, Chen N, Guo H, et al. Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res. 2009; 28:141.

75. Secchiero P, Zorzet S, Tripodo C, et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PLoS One. 2010; 5:e11140.

76. Han Y, Wang Y, Xu Z, et al. Effect of bone marrow mesenchymal stem cells from blastic phase chronic myelogenous leukemia on the growth and apoptosis of leukemia cells. Oncol Rep. 2013; 30:1007–1013.

77. Yuan Y, Chen D, Chen X, Shao H, Huang S. Human umbilical cord-derived mesenchymal stem cells inhibit proliferation but maintain survival of Jurkat leukemia cells in vitro by activating Notch signaling. Nan Fang Yi Ke Da Xue Xue Bao. 2014; 34:441–447.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download