Abstract
For patients with newly diagnosed type 2 diabetes mellitus (T2DM), lifestyle modifications including medical nutrition therapy, weight control, physical activity, smoking cessation, and avoidance of alcohol abuse should be initiated. Metformin must be considered as the first-line oral glucose-lowering therapy, but other drugs such as dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 (SGLT-2) inhibitors, thiazolidinediones, glucagon-like peptide 1 receptor agonists, sulfonylureas, glinides, α-glucosidase inhibitors, and insulin can be considered based on patient circumstances. If the initial HbA1c level of a patient is ≥ 7.5% or the HbA1c target is not achieved within three months of initiating monotherapy, dual combination therapy can be considered. If the HbA1c target is not achieved within 3 months of initiating dual therapy, a third agent with a complementary mechanism of action can be added for triple combination therapy. In addition, evidence from large clinical studies assessing cardiovascular outcomes following the use of SGLT-2 inhibitors in T2DM patients with cardiovascular risk factors have been incorporated into the updated recommendations.
Figures and Tables
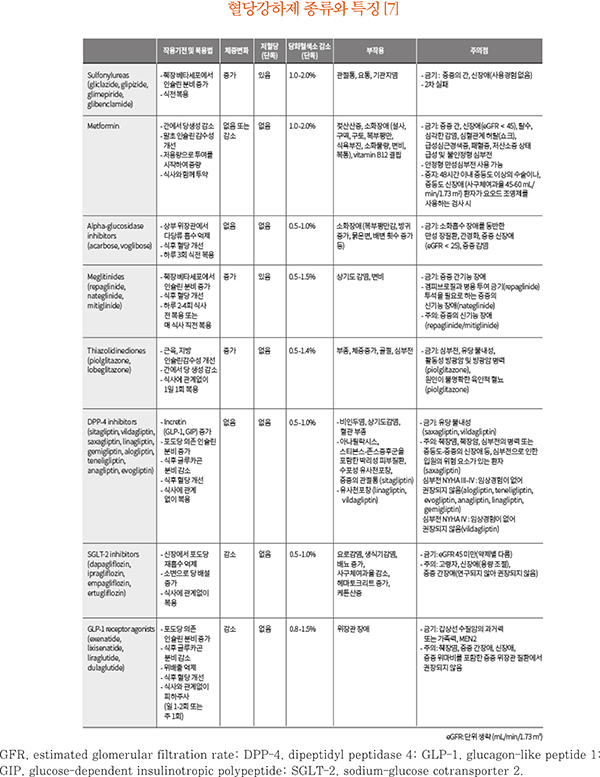
Fig. 1
Adapted from the 2019 treatment guideline for diabetes. Seoul: SeoulMedcus; 2019. p57-64 [7].
Antihyperglycemic therapy algorithm for adult patients with type 2 diabetes mellitus (T2DM). For newly diagnosed T2DM patients, begin with lifestyle modifications at the time of diagnosis and maintain these for the duration of treatment. If the HbA1c target is not achieved within 3 months, then an antihyperglycemic agent should be initiated promptly. Metformin monotherapy is the preferred first choice. But if there are contraindications for metformin or side effects, then consider other monotherapy options such as a dipeptidyl peptidase 4 inhibitor (DPP-4i), sodium-glucose cotransporter 2 inhibitor (SGLT-2i), thiazolidinedione (TZD), glucagon-like peptide 1 receptor agonists (GLP-1 RAs), sulfonylurea (SU), α-glucosidase inhibitor (α-Gi), or insulin as the initial therapy according to the patient's condition. If the initial HbA1c level is ≥ 7.5% or the HbA1c target is not achieved within 3 months of monotherapy, dual combination therapy can be considered. If the HbA1c target is not achieved within 3 months after commencing dual therapy, then proceed to triple combination therapy.
CV, cardiovascular; GLN, glinide.
Efficacy (green), CV benefit (blue), hypoglycemia risk (red), and body weight changes (yellow*) were assigned ratings for low, intermediate, or high (body weight changes*; decrease, neutral, or increase); the scale bar is not constructed according to strict definitions but should be used as a guide for clinical decisions.
*Body weight changes: decrease, neutral, or increase, †GLN can be used as dual combination therapy with metformin, TZD, α-Gi, or insulin or as a triple combination therapy with metformin and α-Gi, metformin and TZD, or metformin and insulin.

References
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998; 352:854–865.
2. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S90–S102.
3. Yoon KH, Shin JA, Kwon HS, Lee SH, Min KW, Ahn YB, Yoo SJ, Ahn KJ, Park SW, Lee KW, Sung YA, Park TS, Kim MS, Kim YK, Nam MS, Kim HS, Park IeB, Park JS, Woo JT, Son HY. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in Korean drug-naïve type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J. 2011; 35:26–33.

4. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of clinical outcomes and adverse events associated with glucoselowering drugs in patients with type 2 diabetes: a metaanalysis. JAMA. 2016; 316:313–324.

5. Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011; 154:602–613.

6. Qaseem A, Barry MJ, Humphrey LL, Forciea MA. Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017; 166:279–290.

7. Ko SH. Oral hypoglycemic agents for patients with type 2 diabetes mellitus. Korean Diabetes Association. 2019 treatment guideline for diabetes. 6th ed. Seoul: SeoulMedcus;2019. p. 57–64.
9. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. updated 2019 Jul 25. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm.
10. Qian D, Zhang T, Zheng P, Liang Z, Wang S, Xie J, Zhao L, Zhang Y, Situ B. Comparison of oral antidiabetic drugs as add-on treatments in patients with type 2 diabetes uncontrolled on metformin: a network meta-analysis. Diabetes Ther. 2018; 9:1945–1958.

11. Zaccardi F, Dhalwani NN, Dales J, Mani H, Khunti K, Davies MJ, Webb DR. Comparison of glucose-lowering agents after dual therapy failure in type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018; 20:985–997.

12. Lee CM, Woodward M, Colagiuri S. Triple therapy combinations for the treatment of type 2 diabetes-a network meta-analysis. Diabetes Res Clin Pract. 2016; 116:149–158.

13. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128.

14. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ. EMPA-REG OUTCOME® Investigators. Empagli flozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease-results From EMPA-REG OUTCOME®. Circ J. 2017; 81:227–234.

15. Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, Neal B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016; 4:411–419.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download