This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Acute infectious diarrhea (AID) is a commonly observed condition globally. Several studies recommend against the use of empiric antibiotic therapy for AID, except in some cases of travelers' diarrhea. However, many physicians prescribe antimicrobial agents for AID. We aimed to determine the rate of antibiotic use and the associated prescription patterns among adults with AID.
Materials and Methods
This population-based, retrospective epidemiological study was performed using Korean National Health Insurance claims data from 2016 to 2017. The study population comprised adults (age ≥18 years) who had visited clinics with AID-related complaints. Exclusion criteria were the presence of Crohn's disease, ulcerative colitis, irritable bowel syndrome, and other non-infectious forms of colitis. Patients who underwent surgery during admission were also excluded.
Results
The study population comprised 1,613,057 adult patients with AID (767,606 [47.6%] men). Young patients (age 18 – 39 years) accounted for 870,239 (54.0%) of the study population. Overall, 752,536 (46.7%) cases received antibiotic prescriptions. The rate of antibiotic administration tended to be higher among elderly patients (age ≥65 years) than among younger patients (49.5% vs. 46.4%, P <0.001). The antibiotics most frequently prescribed in both monotherapy and combination regimens were fluoroquinolones (29.8%), rifaximin (26.8%), second-generation cephalosporins (9.2%), third-generation cephalosporins (7.3%), trimethoprim/sulfamethoxazole (5.5%), and β-lactam/β-lactamase inhibitors (5.3%). Patients who visited tertiary care hospitals had lower rates of antibiotic therapy (n = 14,131, 41.8%) than did those visiting private clinics (n = 532,951, 47.1%). In total, 56,275 (62.3%) admitted patients received antibiotic therapy, whereas outpatients had lower rates of antibiotic prescription (n = 694,204, 46.0%).
Conclusion
This study revealed differences between the antibiotics used to treat AID in Korea and those recommended by the guidelines for AID treatment. Multifaceted efforts are necessary to strengthen physicians' adherence to published guidelines.
Keywords: Dysentery, Anti-bacterial agents, Epidemiology, Diarrhea, Gastroenteritis
Introduction
Acute infectious diarrhea (AID) is a commonly reported condition among outpatients, worldwide [
1]. Several studies have demonstrated that antibiotic use is non-ideal for AID treatment, except in some cases of travelers' diarrhea or severe AID. Since bacteria are the causative organisms in only some cases of acute diarrheal disease, several cases often show improvement without antibiotic use [
123]. An epidemiological study reported that most cases of community-acquired diarrhea are caused by viral pathogens; therefore, antibiotic treatment would not shorten the overall duration [
1]. However, antibiotics are used frequently for AID. Inappropriate antibiotic use can lead to increased antibiotic resistance and medical costs, while also causing antibiotic-associated adverse events. While data on gastrointestinal infections are being collected by the Korea Centers for Disease Control and Prevention, the current status of antibiotic prescription for AID has not been researched. Accordingly, the objective of this study was to identify the current rates of antibiotic use and prescriptions for AID among Korean adults.
Materials and Methods
This study used National Health Insurance claims data for the assessment of the current status of antibiotic use among Korean adults diagnosed with AID. The health insurance system in Korea is a public insurance system that covers all Korean citizens, and runs independently of healthcare providers and private insurance companies. In the National Health Insurance system, there is no discrimination in the provision of access to healthcare resources based on patients' income levels [
4]. This study targeted adults aged 18 years or older, and National Health Insurance data pertaining to claims for AID in the three previous years (January 2015 to December 2017) were obtained from the Health Insurance Review and Assessment Service (HIRA). Patients who were diagnosed for the first time in 2015 and for whom subsequent claims were made under the same diagnostic code in 2016 and/or 2017 were excluded. Accordingly, the actual analysis included only new claim cases pertaining to AID-related codes in 2016 and 2017.
The HIRA database consists of diagnoses coded using the Korean Classification of Disease (7th edition). Among the health insurance claims data, we included those regarding the infectious diarrhea-related codes, comprising A020 (Salmonella enteritis), A044 (other intestinal Escherichia coli infection), A045 (Campylobacter enteritis), A046 (enteritis due to Yersinia enterocolitica), A049 (bacterial intestinal infection, unspecified), A060 (acute amoebic dysentery), A079 (protozoal intestinal disease, unspecified), A080 (rotaviral enteritis), A081 (acute gastroenteropathy due to Norwalk agent), A082 (adenoviral enteritis), A0830 (other viral enteritis), A0831 (astroviral gastroenteritis), A0838 (other viral enteritis), A084 (viral intestinal infection, unspecified), A090 (other and unspecified gastroenteritis and colitis of infectious origin), and A099 (gastroenteritis and colitis of unspecified origin). Of the entries that contained the diagnostic codes listed above, those that also contained diagnostic codes for inflammatory diarrheal diseases, such as K50 (Crohn's disease [regional enteritis]), K51 (ulcerative colitis), K52 (other noninfective gastroenteritis and colitis), and K58 (irritable bowel syndrome), were excluded.
Regardless of the diagnostic code rank, cases that included any of the aforementioned codes in the entire diagnosis were included in the analysis. However, patients who underwent surgery during the period in which the claim was made for the diagnostic code were excluded. Inpatient cases for whom the aforementioned codes were entered on the first day of hospitalization were included, while those for whom diagnostic codes were entered during hospitalization were excluded. In addition, we included cases for which the specialty of the physician filing the claim was internal medicine, family medicine, emergency medicine, or general practice. The medical facilities were classified according to the system provided by the HIRA. Private clinics, hospitals, general hospitals, and tertiary care hospitals were defined as medical institutions with <30 hospital beds, 30 – 39 hospital beds, ≥100 hospital beds and 6 – 9 divisions or departments, and ≥500 hospital beds and ≥20 divisions or departments, respectively.
This study used routinely collected National Health Insurance data for the purposes of insurance claims; thus, ethics approval was not required. All data were analyzed via a chi-square test and population ratio test using SAS Enterprise Guide version 6.1 (SAS Institute Inc., Cary, NC, USA).
Results
Claims were filed for a total of 2,494,737 patients with infectious diarrhea between 2015 and 2017. After excluding 881,680 patients for whom multiple claims were filed for the same diagnostic code in 2015, a total of 1,613,057 patients were included in the analysis (840,443 and 772,614 patients were diagnosed for the first time in 2016 and 2017, respectively). The total numbers of men and women were 767,606 (47.6%) and 845,451 (52.4%), respectively.
Figure 1 shows the distribution of patients' ages and sex ratio.
 | Figure 1 Distribution of adult patients with infectious diarrhea, according to age group and sex.
|
Antibiotic usage rate
In our study population, 752,536 (46.7%) patients used antibiotics.
1. Antibiotic usage rate by age group
The antibiotic usage rates in the 18 – 19, 20 – 29, 30 – 39, 40 – 49, 50 – 59, 60 – 69, 70 – 79, and ≥80 year age groups were 43.6%, 47.6%, 46.6%, 45.3%, 46.6%, 48.0%, 48.9%, and 51.0%, respectively, indicating that the antibiotic usage rate was higher in the older age groups. Using a cut-off age of 65 years, the numbers of those aged <65 and ≥65 years who did not use antibiotics were 791,814 (53.6%) and 68,707 (50.6%), respectively, indicating a higher usage rate among those aged ≥65 years.
2. Antibiotic prescription rate by medical facility type
Figure 2 shows the antibiotic prescription rate by medical institution type. The results showed that the antibiotic prescription rate was higher in private clinics and hospitals than in tertiary care hospitals and general hospitals.
 | Figure 2Frequency of antibiotic prescription for patients with infectious diarrhea, according to medical facility type.
|
3. Antibiotic prescription rate by region
Figure 3 shows the distribution of patients with AID, and that of those who did or did not use antibiotics, by region. The highest number of patients visited facilities in Seoul and Gyeonggi-do, and the proportions of patients who used antibiotics in these places were 47.8% and 42.8%, respectively. The regions with antibiotic usage rates exceeding 50% were Gwangju (57.4%), Jeollabuk-do (61.0%), Jeju-do (57.0%), Chungcheongbuk-do (53.4%), and Jeollanam-do (52.0%). Incheon (38.8%) and Sejong (38.1%) showed relatively low antibiotic usage rates.
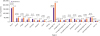 | Figure 3Use of antimicrobial agents among adults with infectious diarrhea, by region.
|
Frequency and duration of antibiotic use
Table 1 shows the frequency and duration of use by antibiotic class. No antibiotic was used exclusively and cases in which two or more antibiotics were used simultaneously were also included. The fluoroquinolone class of antibiotics (n = 276,704, 29.8%) and rifaximin (n=249,012, 26.8%) were most frequently used. Of the 69,194 cases that included all β-lactam/β-lactamase inhibitors and penicillin-class antibiotics, amoxicillin/clavulanate (57.3%) was the most frequently used, while amoxicillin alone was used in 27.7% of cases. Piperacillin/tazobactam and ampicillin/sulbactam were used in 3.6% and 3.4% of cases, respectively. Among the fluoroquinolone class of antibiotics, ciprofloxacin was the most frequently used (70.4%); levofloxacin (6.8%) and moxifloxacin (0.4%) were also used. Among the macrolide class of antibiotics (n=38,653, 4.2%), clarithromycin (37.0%), roxithromycin (24.4%), and azithromycin (AZM) (7.9%) were the most commonly noted. Oral antibiotics were more frequently used compared with intravenous antibiotics, except for clindamycin.
Table 2 shows the frequency and duration of use of combination antibiotic regimens. The fluoroquinolone or cephalosporin class of antibiotics was the most frequently used in combination with aminoglycosides.
Table 1
Frequency and duration of antibiotic prescription for patients with infectious diarrhea

|
Antibiotics |
Frequency, n (%) |
Duration, days (mean ± SD) |
Route of administration |
Age group |
Medical facility type |
Patient status |
|
IV (number of prescriptions) |
PO (number of prescriptions) |
Age <65 yrs, n (%) |
Age ≥65 yrs, n (%) |
Tertiary care hospital, n (%) |
General hospital, n (%) |
Hospital, n (%) |
Private clinic, n (%) |
Inpatient, n (%) |
Outpatient, n (%) |
|
4th generation cephalosporins |
214 (0.0) |
12.08 ± 8.75 |
214 |
|
105 (0.0) |
109 (0.1) |
64 (0.3) |
129 (0.1) |
21 (0.0) |
0 (0.0) |
205 (0.2) |
9 (0.0) |
|
3rd generation cephalosporins |
67,335 (7.3) |
6.02 ± 5.45 |
38,102 |
45,418 |
57,125 (6.8) |
10,210 (11.0) |
4,101 (18.2) |
39,161 (23.3) |
14,465 (13.4) |
9,608 (1.5) |
33,098 (29.3) |
34,237 (4.2) |
|
2nd generation cephalosporins |
85,528 (9.2) |
3.84 ± 3.76 |
3,421 |
83,081 |
78,521 (9.4) |
7,007 (7.6) |
165 (0.7) |
7,156 (4.3) |
9,131 (8.5) |
69,076 (11.0) |
4,164 (3.7) |
81,364 (10.0) |
|
1st generation cephalosporins |
16,953 (1.8) |
3.39 ± 3.41 |
5,073 |
12,131 |
15,023 (1.8) |
1,930 (2.1) |
174 (0.8) |
1,809 (1.1) |
2,290 (2.1) |
12,680 (2.0) |
2,121 (1.9) |
14,832 (1.8) |
|
Fluoroquinolones |
276,704 (29.8) |
4.98 ± 5.99 |
38,828 |
264,741 |
250,621 (30.0) |
26,083 (28.1) |
12,099 (53.7) |
60,371 (35.9) |
36,436 (33.8) |
167,798 (26.6) |
37,274 (33.0) |
239,430 (29.4) |
|
Aminoglycosides |
32,944 (3.6) |
4.48 ± 6.91 |
32,944 |
|
29,097 (3.5) |
3,847 (4.1) |
63 (0.3) |
2,552 (1.5) |
4,978 (4.6) |
25,351 (4.0) |
4,026 (3.6) |
28,918 (3.6) |
|
Macrolides |
38,653 (4.2) |
11.55 ± 12.27 |
14,822 |
28,653 |
32,902 (3.9) |
5,751 (6.2) |
2,699 (12.0) |
15,142 (9.0) |
7,499 (7.0) |
13,313 (2.1) |
15,189 (13.4) |
23,464 (2.9) |
|
β-lactam/β-lactamase inhibitors |
49,186 (5.3) |
5.35 ± 6.22 |
6,018 |
44,379 |
44,227 (5.3) |
4,959 (5.3) |
741 (3.3) |
5,053 (3.0) |
5,418 (5.0) |
37,974 (6.0) |
5,031 (4.5) |
44,155 (5.4) |
|
Metronidazole |
26,169 (2.8) |
9.29 ± 12.73 |
287 |
26,008 |
23,197 (2.8) |
2,972 (3.2) |
575 (2.6) |
2,721 (1.6) |
2,067 (1.9) |
20,806 (3.3) |
1,960 (1.7) |
24,209 (3.0) |
|
Tetracycline |
4,469 (0.5) |
12.29 ± 12.74 |
3 |
4,467 |
3,826 (0.5) |
643 (0.7) |
261 (1.2) |
781 (0.5) |
584 (0.5) |
2,843 (0.5) |
827 (0.7) |
3,642 (0.5) |
|
Carbapenems |
1,243 (0.1) |
19.43 ± 16.58 |
1,243 |
|
465 (0.1) |
778 (0.8) |
161 (0.7) |
786 (0.5) |
291 (0.3) |
5 (0.0) |
1,200 (1.1) |
43 (0.0) |
|
Glycopeptides |
834 (0.1) |
18.53 ± 23.73 |
241 |
604 |
541 (0.1) |
293 (0.3) |
143 (0.6) |
569 (0.3) |
66 (0.1) |
56 (0.0) |
429 (0.4) |
405 (0.1) |
|
Trimethoprim/sulfamethoxazole |
51,405 (5.5) |
3.77 ± 4.85 |
30 |
51,395 |
46,885 (5.6) |
4,520 (4.9) |
89 (0.4) |
1,702 (1.0) |
1,422 (1.3) |
48,192 (7.7) |
606 (0.5) |
50,799 (6.2) |
|
Clindamycin |
588 (0.1) |
9.69 ± 13.71 |
495 |
176 |
460 (0.1) |
128 (0.1) |
33 (0.2) |
119 (0.1) |
135 (0.1) |
301 (0.1) |
179 (0.2) |
409 (0.1) |
|
Penicillins |
20,008 (2.2) |
7.44 ± 9.57 |
191 |
19,835 |
17,872 (2.1) |
2,136 (2.3) |
244 (1.1) |
1,279 (0.8) |
1,593 (1.5) |
16,892 (2.7) |
369 (0.3) |
19,639 (2.4) |
|
Rifaximin |
249,012 (26.8) |
4.56 ± 6.22 |
|
249,012 |
228,567 (27.4) |
20,445 (22.0) |
888 (3.9) |
28,602 (17.0) |
20,988 (19.5) |
198,534 (31.5) |
6,235 (5.5) |
242,777 (29.8) |
|
Othersa
|
7,363 (0.8) |
5.66 ± 10.10 |
7,313 |
50 |
6,342 (0.8) |
1,021 (1.1) |
21 (0.1) |
230 (0.1) |
383 (0.4) |
6,729 (1.1) |
179 (0.2) |
7,184 (0.9) |
|
Total |
928,608 (100) |
|
|
|
835,776 (100) |
92,832 (100) |
22,521 (100) |
168,162 (100) |
107,767 (100) |
630,158 (100) |
113,092 (100) |
815,516 (100) |

Table 2
Prescription of antibiotic combination regimens for patients with infectious diarrhea

|
Antibiotics |
Frequency, n |
Duration, days (mean ± SD) |
|
Cephalosporin + metronidazole |
2,264 |
8.96 ± 7.38 |
|
Fluoroquinolone + metronidazole |
901 |
17.84 ± 17.07 |
|
Carbapenem + metronidazole |
81 |
23.35 ± 15.90 |
|
Penicillin + metronidazole |
3,011 |
10.07 ± 5.95 |
|
β-lactam/β-lactamase inhibitors + metronidazole |
715 |
10.63 ± 10.15 |
|
Cephalosporin + aminoglycosides |
9,828 |
5.13 ± 5.33 |
|
Aminoglycosides + metronidazole |
795 |
6.31 ± 9.48 |
|
β-lactam/β-lactamase inhibitors + aminoglycosides |
3,432 |
6.53 ± 9.98 |
|
Fluoroquinolones + aminoglycosides |
11,447 |
3.75 ± 6.65 |
|
Cephalosporin + clindamycin |
227 |
10.32 ± 8.28 |
|
Fluoroquinolones + clindamycin |
105 |
18.35 ± 14.96 |

1. Current status of antibiotic use by age group
Table 1 shows the current status of antibiotic use among patients aged <65 and ≥65 years. Statistically significant differences were observed among all the antibiotics used (
P <0.001). Patients aged ≥65 years showed a higher frequency of use of broad-spectrum, high-priced antibiotics, such as third and fourth-generation cephalosporins, carbapenems, and glycopeptides. However, they showed a relatively lower frequency of use of fluoroquinolones, rifaximin, second-generation cephalosporins, and trimethoprim/sulfamethoxazole, compared with patients aged <65 years.
2. Current status of antibiotic use by medical facility type
Tables 1 and
3 show the current status of antibiotic use by medical facility type. All the items showed statistically significant differences (
P <0.001). The fluoroquinolone class of antibiotics was most frequently prescribed in tertiary care hospitals, followed by macrolides. Third-generation cephalosporins were most frequently used in general hospitals. Private clinics used second-generation cephalosporins and rifaximin more frequently than did other medical facilities; in contrast, third-generation cephalosporins were used less frequently. Aminoglycosides were more frequently used in private clinics than in tertiary care hospitals or general hospitals. However, aminoglycosides were not frequently used in combination regimens, indicating that private clinics often used aminoglycosides alone.
Table 3
Prescription of antibiotic combination regimens for patients with infectious diarrhea, according to the type of medical facility

|
Antibiotics |
Frequency, n (%) |
Tertiary care hospital, n (%) |
General hospital, n (%) |
Hospital, n (%) |
Private clinic, n (%) |
|
Cephalosporin + metronidazole |
2,264 (0.3) |
260 (1.8) |
1,123 (0.9) |
519 (0.6) |
362 (0.1) |
|
Fluoroquinolone + metronidazole |
901 (0.1) |
87 (0.6) |
444 (0.4) |
236 (0.3) |
134 (0.0) |
|
Carbapenem + metronidazole |
81 (0.0) |
10 (0.1) |
52 (0.0) |
18 (0.0) |
1 (0.0) |
|
Penicillin + metronidazole |
3,011 (0.4) |
136 (1.0) |
828 (0.7) |
422 (0.5) |
1,625 (0.3) |
|
β-lactam/β-lactamase inhibitors + metronidazole |
715 (0.1) |
56 (0.4) |
273 (0.2) |
171 (0.2) |
215 (0.0) |
|
Cephalosporin + aminoglycosides |
9,828 (1.3) |
58 (0.4) |
1,960 (1.6) |
2,205 (2.7) |
5,605 (1.1) |
|
Aminoglycosides + metronidazole |
795 (0.1) |
5 (0.0) |
60 (0.0) |
156 (0.2) |
574 (0.1) |
|
β-lactam/β-lactamase inhibitors + aminoglycosides |
3,432 (0.5) |
30 (0.2) |
440 (0.4) |
636 (0.8) |
2,326 (0.4) |
|
Fluoroquinolones + aminoglycosides |
11,447 (1.5) |
149 (1.1) |
1,083 (0.9) |
1,799 (2.2) |
8,416 (1.6) |
|
Cephalosporin + clindamycin |
227 (0.0) |
28 (0.2) |
104 (0.1) |
52 (0.1) |
43 (0.0) |
|
Fluoroquinolones + clindamycin |
105 (0.0) |
15 (0.1) |
53 (0.0) |
27 (0.0) |
10 (0.0) |
|
Other regimens |
719,730 (95.6) |
13,297 (94.1) |
116,857 (94.8) |
75,936 (92.4) |
513,640 (96.4) |
|
Total |
752,536 (100) |
14,131 (100) |
123,277 (100) |
82,177 (100) |
532,951 (100) |

3. Current status of antibiotic use by patient status (inpatient versus outpatient)
Among inpatients, 56,275 of 90,380 patients (62.3%) used antibiotics, while 696,261 of 1,522,677 outpatients (45.7%) used antibiotics.
Table 1 shows the current status of antibiotic use among inpatients and outpatients. All items, apart from those with glycopeptides (
P = 0.41), showed statistically significant differences (
P <0.001). Compared to outpatients, inpatients used third-generation cephalosporins more frequently. Inpatients also had a higher frequency of macrolide, carbapenem, and glycopeptide use than outpatients did. Outpatients used second-generation cephalosporins, metronidazole, trimethoprim/sulfamethoxazole, penicillin-class antibiotics, and rifaximin more frequently.
Discussion
The results of this study showed that approximately half of all patients used antibiotics and that various classes of antibiotics other than those recommended in the treatment guidelines were also frequently used. The treatment guidelines for AID that have been published in Korea include the Clinical Guideline for the Diagnosis and Treatment of Gastrointestinal Infections that was published in 2010 [
3] and revised in 2019 [
5]. Both guidelines recommend the use of antibiotics for travelers' diarrhea or diarrheal disease with moderate-to-severe symptoms [
35]. The guidelines also recommend the use of fluoroquinolones, AZM, or rifaximin [
35]. The findings of the present study showed that various types of antibiotics are currently being used for AID in Korea, with fluoroquinolones and rifaximin showing the highest usage rates, while AZM showed a low frequency of use.
According to a report published by the Korea Centers for Disease Control and Prevention in 2017 on reported culture-positive cases of gastrointestinal infection in Korea, viral and bacterial infections accounted for 59% and 40.5% of all cases, respectively. In decreasing order of frequency, the causative pathogens that have been reported include
Salmonella, Clostridium perfringens, and
Campylobacter [
6]. Although fluoroquinolones, such as ciprofloxacin, are recommended as empiric antibiotics, macrolides, such as AZM, are recommended for
Campylobacter infections due to the increasing trend of resistance to fluoroquinolones [
78910]. Rifaximin is a safe drug, the efficacy of which is similar to that of fluoroquinolones; thus, its use has increased in recent times [
11].
However, most patients with acute watery diarrhea do not require antibiotic therapy [
5]. In most cases, the cause is viral, and even in cases caused by bacteria, most patients show improvements naturally without any specific treatment [
21213]. In the present study, antibiotics were used in approximately 47% of AID cases, suggesting that unnecessary antibiotics were used too often. In particular, the antibiotic prescription rate was higher in private clinics than in tertiary care hospitals. This is believed to be due to the fact that private clinics tend to have limited laboratory tests, and experience a greater level of pressure when patients do not show improvements in symptoms within a short period of time.
With respect to the current status of use of combination antibiotic regimens, cephalosporins or fluoroquinolones were often combined with aminoglycosides. The use of such combination antibiotic regimens may be helpful in the treatment of moderate-to-severe Gram-negative infections, but the use of aminoglycosides is not generally recommended for simple cases of AID [
214].
In the present study, patients aged ≥65 years used antibiotics more frequently, which might be attributable to the fact that older patients typically have a higher severity of AID and consequently a higher mortality rate [
15]. The average length of hospital stay among patients aged ≥75 years with diarrheal disease was found to be 7.4 days, which was approximately 3 days longer than the 4.1 days observed among patients aged 20 – 49 years [
16].
The present study had some limitations. First, we did not consider the exact diagnosis and clinical features of individual patients, and our analyses were based on secondary data obtained from HIRA that covers the entire population. Second, the AID-related diagnostic codes included main diagnoses and sub-diagnoses, and only a small number of patients were excluded; however, we cannot deny that duplicate patients with other diseases could be present. Third, since there is no separate diagnostic code for travelers' diarrhea, patients with travelers' diarrhea could not be assessed separately. Fourth, there is a lack of research data that can be used to compare the prevalence of AID and antibiotic usage rates for validity testing. Fifth, there may have been cases in which diagnostic codes unrelated to the actual patient diagnosis may have been entered to avoid cutbacks on healthcare use claims by the HIRA; however, such cases could not be excluded since the medical records of the patients could not be checked.
Despite these limitations, the present study is significant in that, to the best of our knowledge, it is the first to investigate the current status of antibiotic prescriptions for AID in Korea. In our study, we found that about half of AID patients received prescriptions for antibiotics, against the recommendations of the AID guidelines. Also, the study describes the recent epidemiology of AID in Korea.
In conclusion, the present study demonstrates a disparity in the rates of antibiotic use in current clinical practice and those mentioned in current guidelines for AID in Korea. The findings also indicate the need for each medical facility to review the appropriateness of the use of antibiotics for AID treatment and educate clinicians on the proper use of antibiotics in accordance with existing guidelines. Future studies should focus on the clinical features of patients and analysis of causative pathogens.
ACKNOWLEDGMENTS
We would like to thank Jun-Pyo Myong and colleagues, for analyzing the data.
References
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016; 111:602–622.

2. Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017; 65:1963–1973.

3. The Korean Society of Infectious Diseases, Korean Society for Chemotherapy, The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of gastrointestinal infections. Infect Chemother. 2010; 42:323–361.
4. Yoon YK, Kim EJ, Chun BC, Eom JS, Park DW, Sohn JW, Kim MJ. Prescription of antibiotics for adults hospitalized with community-acquired pneumonia in Korea in 2004: a population-based descriptive study. Respirology. 2012; 17:172–179.

5. Kim YJ, Park KH, Park DA, Park J, Bang BW, Lee SS, Lee EJ, Lee HJ, Hong SK, Kim YR. Guideline for the antibiotic use in acute gastroenteritis. Infect Chemother. 2019; 51:217–243.

7. Eurosurveillance editorial team. European union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food 2012 published. Euro Surveill. 2014; 19:20748.
8. Zaidi MB, McDermott PF, Campos FD, Chim R, Leon M, Vazquez G, Figueroa G, Lopez E, Contreras J, Estrada-Garcia T. Antimicrobial-resistant Campylobacter in the food chain in Mexico. Foodborne Pathog Dis. 2012; 9:841–847.
9. Serichantalergs O, Pootong P, Dalsgaard A, Bodhidatta L, Guerry P, Tribble DR, Anuras S, Mason CJ. PFGE, Lior serotype, and antimicrobial resistance patterns among Campylobacter jejuni isolated from travelers and US military personnel with acute diarrhea in Thailand, 1998-2003. Gut Pathog. 2010; 2:15.

10. Cho IN, Yim J, Lee Y, Kim MS, Seo Y, Chung HS, Yong D, Jeong SH, Lee K, Chong Y. Trends in isolation and antimicrobial susceptibility of enteropathogenic bacteria in 2001-2010 at a Korean Tertiary Care Hospital. Ann Clin Microbiol. 2013; 16:45–51.

11. Riddle MS, Connor P, Fraser J, Porter CK, Swierczewski B, Hutley EJ, Danboise B, Simons MP, Hulseberg C, Lalani T, Gutierrez RL, Tribble DR. TrEAT TD Study Team. TrEAT TD Study Team. Trial evaluating ambulatory therapy of travelers' diarrhea (TrEAT TD) study: a randomized controlled trial comparing 3 single-dose antibiotic regimens with loperamide. Clin Infect Dis. 2017; 65:2008–2017.

12. Acree M, Davis AM. Acute diarrheal infections in adults. JAMA. 2017; 318:957–958.

13. Lübbert C. Antimicrobial therapy of acute diarrhoea: a clinical review. Expert Rev Anti Infect Ther. 2016; 14:193–206.

14. Lamb HM, Ormrod D, Scott LJ, Figgitt DP. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs. 2002; 62:1041–1089.
15. Mounts AW, Holman RC, Clarke MJ, Bresee JS, Glass RI. Trends in hospitalizations associated with gastroenteritis among adults in the United States, 1979-1995. Epidemiol Infect. 1999; 123:1–8.

16. Trinh C, Prabhakar K. Diarrheal diseases in the elderly. Clin Geriatr Med. 2007; 23:833–856. vii








 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download