Introduction
Keratinocytes constitute the major cellular component in the epidermis and play critical roles in the process of wound healing. They play a major part in the complex mechanisms involved in the initiation, continuation, and completion of wound healing. The properties of keratinocytes in chronic wounds differ in different locations and situations. Normally, keratinocytes are responsible for the proliferative and regenerative capacity of the epidermis by providing a continuous supply of proliferative cells. The re-epithelization of the wounded skin occurs by the rapid and coordinated migration of keratinocytes [
1].
The keratinocytes present at the edges of the non-healing chronic wounds are different from the healthy and normal keratinocytes. A successful wound closure requires a cross-talk between the keratinocytes and other cell types involved in wound healing [
1]. Keratinocytes may also play a role in the extracellular matrix reorganization [
2].
Owing to their diverse role in the healing process, the keratinocytes have been chosen in the current study to evaluate the wound healing benefits of the traditional medicines.
Traditional medicinal systems of India such as Ayurveda and folk medicine have been using such effective medicinal preparations to treat wounds, and have claimed that these medications not only act as anti-inflammatory, anti-microbial reagents, but also have rejuvenating and regenerating effects.
One such effective alternative treatment strategy is the use of honey, ghee, and
Glycyrrhiza glabra (GG), and a folk medicinal preparation using
Nerium indicum (NI), which is effectively practiced to treat various types of wounds since many years in India [
3]. Several studies have shown the wound healing benefits of individual application of ghee [
4] or honey [
56]. The wound healing, anti-ulcer, anti-inflammatory, and skin regeneration activity of GG is also documented by an earlier research [
7]. NI is another herb used to treat wounds in the folk medicine [
8]. Our previously published report on wound healing in animal models using these traditional medicines singly and in combinations provided valuable evidences on microenvironmental changes in the wound site, thus proving the efficacy of these medicines [
3]. The influence of these traditional medicines singly and in combination on the fibroblasts have also been experimented upon [
9].
However, less is known about the impact of these traditional medicines on keratinocytes in the context of wound healing. The present study was therefore designed to evaluate the influence of these traditional medicines on the activities of keratinocytes, such as proliferation and migration, which are critical in wound healing process. The study may also aid in supporting the possibility of using the traditional medicines in wound healing, especially in managing the medically challenging wounds such as diabetic foot ulcers, pressure sores, ischemic ulcers, etc.
Go to :

Discussion
Keratinocytes are the chief cellular constituents of the epidermis and have numerous critical responsibilities in the process of wound healing. They are involved in the intricate mechanisms of initiation, maintenance, and completion of wound healing [
1].
Owing to its significant role in wound healing, keratinocytes (HaCaT cells) have been chosen in the current study to appreciate the healing benefits of the traditional medicines.
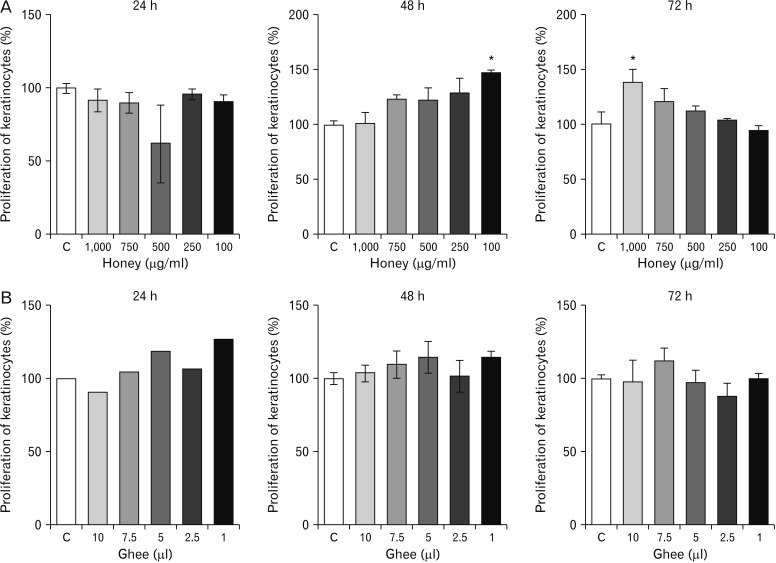
Measuring viability and growth of the cells, i.e., keratinocytes when treated with the different concentrations of the test materials is a positive step to test their efficiency. The accurate method to evaluate the same is the use of MTT assay [
12]. It provides a precise determination of the alterations in the cell proliferation rate [
14].
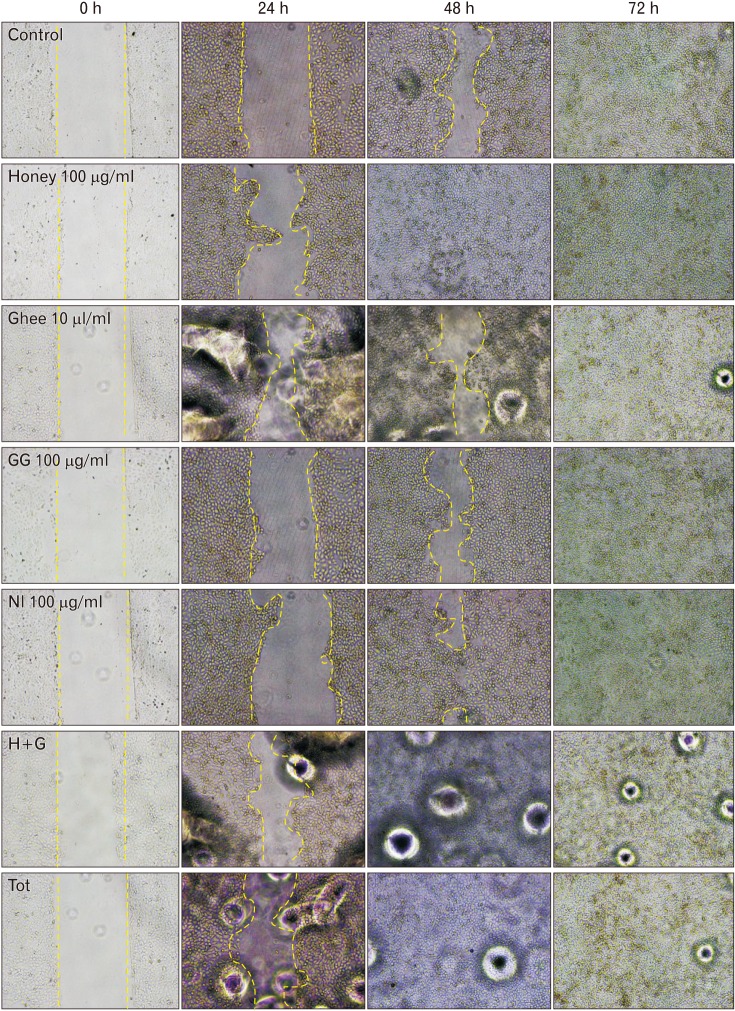
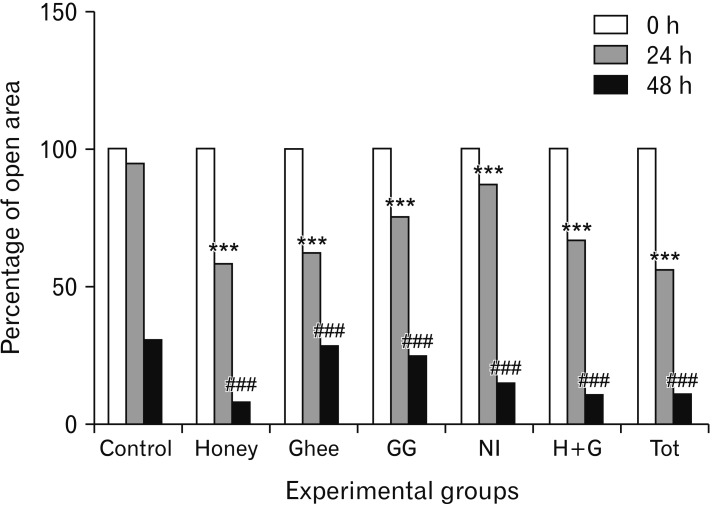
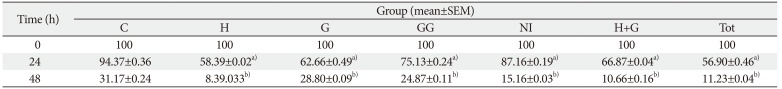
The scratch wound healing assay is yet another important method used to portray the cell proliferation and migration [
15]. Both the methods are therefore employed in the present study to understand the influence of the traditional medicines on the keratinocytes.
The exposure of honey was previously found to stimulate wound healing in the rat dermal fibroblasts (RDFs) as indicated by the scratch assay. Among the three kinds of honey used, i.e., Acacia (
Robinia pseudacacia), Buckwheat (
Fagopyrum sp.), and Manuka (
Leptospermum scoparium); Acacia and buckwheat honey showed significant results compared to Manuka honey. It indicates that the different types of honey may present different wound healing properties [
16].
Our previous reports on the MTT assay using RDFs revealed that honey neither showed any proliferative nor cytotoxic effect on the fibroblasts. The scratch assay revealed better migration of the fibroblasts treated with honey. But the findings were not statistically significant [
9]. Honey-treated keratinocytes, on the contrary, showed proliferative effects after 48 and 72 hours of treatment as indicated in the present study. The scratch assay conducted on honey-treated keratinocytes showed a significant narrowing of the wound. It denotes that honey has better proliferative effects on keratinocytes compared to the fibroblasts.
Studies have confirmed that honey induces low cytotoxicity in keratinocytes. Further, the honey driven wound repair goes through activation of keratinocyte re-epithelization [
17]. Greater the duration of exposure to honey, a greater rate of proliferation as was observed in the present study.
The proliferative benefits of honey could be attributed to the involvement of specific mechanisms that are still obscure. Recent studies have however shown that honey has a chemoattractant effect [
17]. It could be responsible for the improved proliferation and migration of the keratinocytes as was observed in the present study.
Honey is responsible for the enhanced expression of syndecan-4 (transmembrane heparin sulfate) and matrix metalloproteinase (MMP)-9. MMPs are known to play essential roles in wound repair linked cell migration [
17]. Honey also aids in the activation of proteins involved in the proliferation and cytoskeletal rearrangement. Honey activates cyclin-dependent kinase 2, a cyclin dependent kinase known to be involved in G1-S transition and keratinocyte proliferation. Focal adhesion kinase and rasGAP SH3 binding protein 1, both involved in cell locomotion are also shown to be activated by honey. Vasodilator-stimulated phosphoprotein, integrin-β3, CDC25C, and p42/44 mitogen activated protein kinase are among the other peptides related to cytoskeletal and cell cycle dynamics. They have also shown variable amount of activation on exposure to honey [
17]. Honey is also known to play a prime role in the induction of epithelial mesenchymal transition (EMT). The EMT is essential for the keratinocyte re-epithelization as is observed in wound healing [
17].
The abovementioned factors and mechanisms support the observations made in the present study. It further reaffirms the proliferative and migratory benefits of honey and reinstates the belief that honey increases the wound repair capabilities of keratinocytes.
The polyunsaturated fatty acid (PUFA) present in the ghee is capable of controlling cell to cell interaction and intracellular signal transduction [
18]. The
n-3 and
n-6 PUFA also enable the proliferation of epithelial cells
in vitro which serves as an essential step in wound repair [
19].
Ghee showed proliferative effects on the keratinocytes as indicated in the current study. The cells were proliferative when exposed to ghee for shorter durations. The decrease in the proliferative implications of these cells on exposure to longer duration may be probably due to the immiscible nature of ghee which formed an oily layer of film on the culture medium, thereby interfering with the CO
2 and oxygen ratio in the culture medium, which has an adverse effect on cell survival and growth. Similar observations were made by the fibroblasts treated with ghee in previous reports [
9]. The scratch assay, however, showed significant wound closure in ghee treated cells. It indicates that ghee can increase the wound closure abilities of the keratinocytes by influencing its migration. The actual mechanisms of action are however unclear and further needs to be explored.
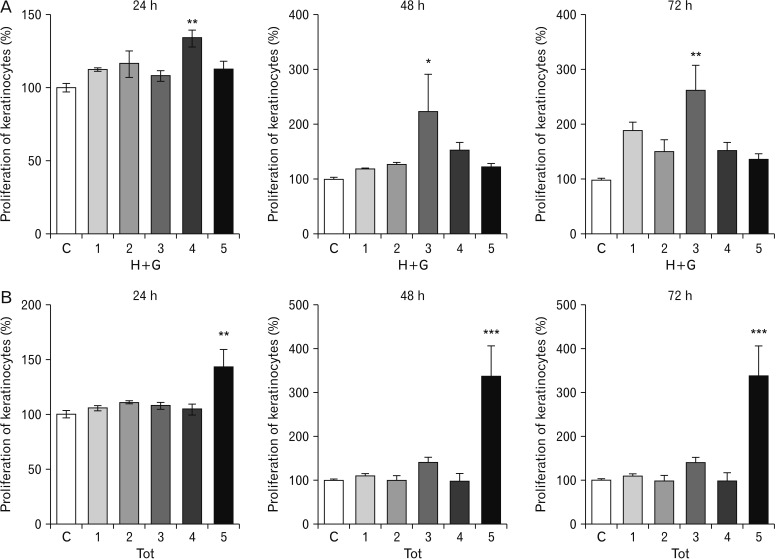
The H+G treated cells on prolonged exposure also showed better proliferative and migratory effects.
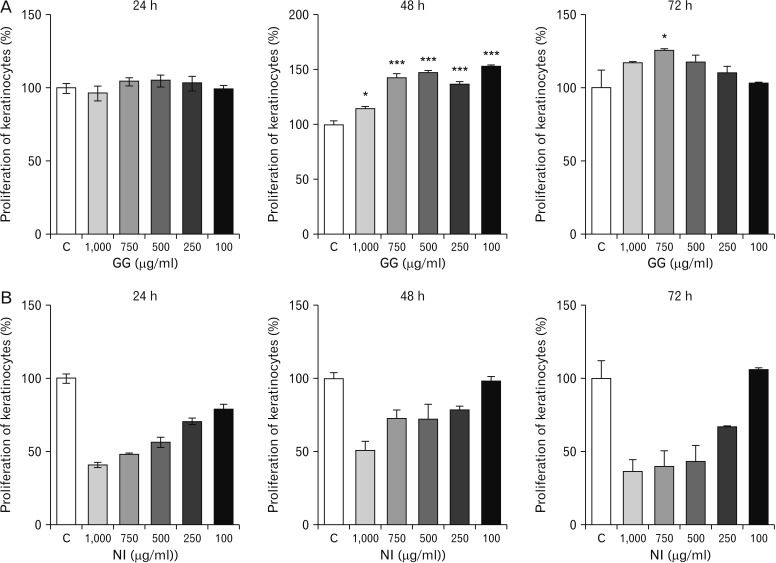
GG had a positive proliferative effect on the keratinocytes. Greater the dose, higher was the proliferation rate. Further, the migration of the keratinocytes was also influenced by GG on exposure to longer duration as indicated by the scratch assay. Research has revealed that GG inhibits the proliferation of abnormal cells, and is anti-carcinogenic in nature. However, the exact mechanisms influencing these activities are still being investigated [
20]. On the contrary, in the present study, GG has shown a positive influence on cell proliferation. A similar observation was made in the previous study on fibroblasts [
9]. This indicates that GG has a variable influence on the normal and abnormal cells.
Although GG has shown to improve the rate of cell proliferation and migration of keratinocytes and thereby improve wound healing, the underlying mechanisms are obscure. However, it could be suggested that GG aids in the activation of proteins involved in the proliferation and cytoskeletal rearrangement, and thus improves healing. The increased duration of exposure to GG also has a positive impact on the increased activities of the keratinocytes. Longer the duration of exposure, greater could be the activation of specific growth factors/proteins aiding in healing that needs to be further investigated. The antioxidant and rejuvenating effects of some of the constituents of GG such as glycyrrhizin and glabridin may also aid in improving the wound healing abilities of keratinocytes [
21].
NI, on the contrary, was cytotoxic to keratinocytes in all the calculated higher doses as indicated by the MTT assay. The lower doses of NI neither showed proliferative nor toxic effects. The migration of the keratinocytes treated with NI was however significantly improved compared to control as shown by the scratch assay. It suggests that NI may have a varied influence on the activity of keratinocytes at the wound site at particular dose. Similar observation was made in the previous study on fibroblasts [
9]. Thus, it could be postulated that NI favors cellular migration, but is anti-proliferative in nature. Further analytical studies are required to reaffirm the same.
The animal experiments conducted in the past revealed that although NI exhibited prolonged inflammatory responses, but was however effective in quickening re-epithelialization and reducing fibrosis at the wound site. Further, the wounds treated with NI also showed increased tensile strength at the wound site compared to other test groups [
3]. It indicates that NI is not entirely toxic. In our current study using the HaCaT cells, NI is found to be toxic indicating the many other intrinsic factors are triggered by the use of NI
in vivo setup which aid in the wound repair. Therefore, there is a need for further evaluation. Conducting the future experiments using lower doses of NI would show positive results.
The Tot treated keratinocytes showed proliferative effects. Significant observations were made at the lower concentrations used, owing to the reduction in the amount of NI present in Tot. The scratch assay employing the keratinocytes also showed significant results.
Studies in the past have focused on the role of keratinocyte-fibroblast interactions in the wound healing process. There is ample evidence that keratinocytes stimulate fibroblasts to synthesize growth factors, which in turn stimulates the keratinocyte proliferation in a double paracrine manner. Moreover, fibroblasts can also acquire a myofibroblast phenotype under the control of keratinocytes. This depends on a finely tuned balance between a pro-inflammatory or a transforming growth factor β-dominated environment [
22]. The use of fibroblast and keratinocyte co-cultures to study the healing efficacy of these natural medicines may, therefore, be beneficial.
The preliminary results obtained from the current study reestablishes the benefits of traditional medicine in healing wounds in general and their influence on the keratinocytes in particular. The study indicates that the test materials used, both singly and in combination have a positive influence on the proliferation and migration of the keratinocytes, an important factor required for wound closure and thereby better wound healing.
Honey and GG had a positive influence on the wound healing capabilities of keratinocytes. Ghee and NI although showed better keratinocyte proliferation and migration, the findings were good only at lower concentrations and shorter durations of exposure owing to their cytotoxic effects. The observations made in the present study may further encourage and instigate newer objectives that would identify and explain the process of wound healing, and thereby glorify the effectiveness of the use of these traditional medicines. Further evaluation is also required to understand the intricate cellular and molecular mechanisms of these test materials which may help us to design the appropriate combination of these test materials in treating medically challenging wounds.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download