Abstract
Melatonin or N-acetyl-5-methoxytryptamine, the fascinating molecule secreted by the pineal gland. Melatonin has a close interaction with hypothalamic-pituitary-gonadal axis. In non-seasonal breeders like rat its exact role in reproduction is controvertible. So it is worth to explore the possible role of melatonin on the onset of puberty in male albino rats. Two groups of male rats aged 5 and 10 days were used for the study. In each group, there were three subgroups, each receiving melatonin for 5 days, 10 days or till the day of descent of testes. Similar subgroups were used as controls. Without handling, animals were observed daily for the onset of puberty. On the day of descent of testes, body weight of the animal was noted, blood was collected, serum was separated and used for radio immunoassay. For histomorphometric analysis, all morphometric measurements were done using an occular micrometer. Volume fraction of seminiferous tubules, intertubular connective tissue of testes, cortex and medulla of thymus were estimated by point count method. In both the age groups melatonin advanced the age on descent of testes, increased the body weight, organ weight. It also increased the serum hormone levels. So, in conclusion this study indicates that exogenous melatonin advances the onset of puberty in male albino wistar rats and this effect is more pronounced in the younger animals.
Melatonin (MT) or N-acetyl-5-methoxytryptamine, the fascinating molecule secreted by the pineal gland, was first identified and isolated in Bovine pineal extracts [1]. MT secretion is high during the dark period which indicates that MT synthesis and release is regulated mainly by the light-dark cycle. There is an evidence that MT may have a role in biologic regulation of circadian rhythms, sleep, mood and aging. Apart from this, MT has several important actions like ability to modulate immune response, quench hydroxyl radical-scavenging of free radicals, influence mutagenesis, carcinogenesis etc. The property of MT as a free radical scavenger increases its importance in the process of aging by way of neuroprotection. MT has a close interaction with hypothalamic-pituitary-gonadal axis [2].
MT has a major physiological role in adult mammals through regulation of seasonal reproduction. The influence of MT on reproductive development begins during the prenatal period, extending into postnatal life [3]. In mammals, MT exhibits an inhibitory effect on the reproductive axis [4]. But, in non-seasonal breeders like rat its exact role in reproduction is controvertible. In adult male rats, the effect of exogenous MT varies from ineffective, antigonadal to progonadal [567]. In rats, MT production is partially controlled by gonadal hormones. In male rats circulating testosterone which induces modifications in tyrosine hydroxylase activity in pineal sympathetic nerve terminals, is necessary to maintain the amplitude of the nocturnal MT peak [8]. Functional relationship and feedback between the pineal gland and the testis is reported to exist [9]. Also, possible involvement of pancreas, insulin-like growth factor 1 (IGF-1) in the action of MT on the reproductive axis cannot be ruled out.
With the above facts it is worth to explore the possible role of MT on the onset of puberty in male albino rats.
Inbred strains of Albino rats bred under controlled lighting using 12 hours of light and 12 hours of dark (12L:12D) cycle as per ethical guidelines were used.
Two groups of male rats aged 5 and 10 days were used for the study. In each group, there were three subgroups, each receiving MT for 5 days, 10 days or till the day of descent of testes. Similar subgroups were used as controls and received the vehicle alone i.e., saline-ethanol.
MT (Sigma, St. Louis, MO, USA) dissolved in salineethanol (9:1 v/v; 0.9% NaCl and 100% ethanol), was prepared fresh and the dose 100 µg/day was injected by subcutaneous route at 15:00 hours. Control pups received injections of vehicle alone.
Without handling, animals were observed daily for the onset of puberty in male rats which is judged by descent of testes and is a conventionally accepted sign for the event [10]. On the day of descent of testes, body weight of the animal was noted, anaesthetized with ether and blood was collected, serum was separated and used for radio immunoassay.
After collecting the blood, animals were sacrificed as per the international ethical guide lines (for animals); the organs testes, epididymides, thymus and pancreas were dissected, weighed. Testes and thymus were fixed in Bouin's fluid for histological studies. Tissues were processed and paraffin blocks were prepared. Sections of 5 µm thickness were cut as described by Farris and Griffith [11]. For every 20 serial sections, only five were selected and the remaining was discarded. The sections were later stained with Ehrlich's hematoxylin and eosin [11].
For morphometric analysis, the cross section of the tubules which showed clear and well demarcated boundaries was selected. All morphometric measurements were done using an Occular micrometer. Volume fraction of seminiferous tubules, intertubular connective tissue of testes, cortex and medulla of thymus were estimated by point count method [12] using the eyepiece graticule. Further, cortico-medullary volume fraction ratio was calculated.
The range of age on descent of testes in control group of rats was from 28.75 to 31.25 days. On treatment with MT for different durations in 5 days and 10-day-old rats the age on descent of testes (onset of puberty) was significantly advanced.
Body weight of control and experimental rats ranged from 30.0 to 44.50 g and 36.0 to 48 g, respectively. MT treatment till descent of testes in 5-day-old rats did not affect any significant result. However, in 10-day-old rats MT treatment till descent of testes significantly increased the body weight.
Testes weight in 5-day-old of rats was significantly increased after MT treatment for shorter duration. On contrary, a non-significant decrease in the testes weight was observed after treatment of MT till descent of testes in both age group of rats. In 10-day-old rats, a significant increase in testes weight was observed only after 5 days of treatment.
Epididymides showed a significant increase in weight after treatment with MT in 5-day-old rats after 5 and 10 days of administration. While in 10-day-old rats significant increase in the weight of epididymides was observed in 5-day treatment group, a non-significant decrease was observed after MT treatment for 10 days and till descent.
The seminiferous tubule diameter was significantly increased in 5-day-old rats after 5 days of MT treatment, while in 10-day-old rats significant increase was observed after 5 days and 10 days of MT treatment. Further, in 5-day-old rats MT treatment for 5 days and 10 days significantly increased the seminiferous tubule volume and decreased the intertubular connective tissue volume, while in 10-day-old rats significant change was observed only in rats receiving MT for 10-day duration (Table 3, Fig. 1).
The in-situ thymus gland exhibited well developed cortex and medulla. The in-situ thymus gland cortical volume in all the treatment groups was significantly higher when compared to control group of rats. Similarly, MT resulted in significant reduction in medulla volume in all treatment groups. Cortico-medullary volume ratio was significantly higher in all MT treatment groups of rats except in 10-day-old rats receiving MT till descent (Table 4, Fig. 2).
The serum LH was significantly higher in rats subjected to MT treatment for 5- and 10-day duration in both the age rats. The same effect was not observed in rats subjected to MT treatment till descent of testes in 5-day-old as well as 10-day-old rats (Fig. 3). Further, the serum testosterone levels were found to be statistically significant in both age groups of rats after treatment with MT except in 10-day-old rats receiving MT till descent (Fig. 4). While, serum GH concentrations ranged from 3.34 to 4.25 ng/ml and 3.93 to 6.43 ng/ml in control and experimental group of rats, respectively. Significantly higher serum GH concentrations were observed after treatment with MT in all the groups (Fig. 5).
Descent of testes normally occurs around 15–50 days of postnatal life in male rats [13], the event is considered as the signal for the onset of puberty. The gain in the body weight of the rat also directs for the earlier sexual maturity. Exogenous MT was shown to have a dose-dependent inhibitory action on sexual maturation when given daily from 20 to 40 days of age in the laboratory rat [5]. In contrast, when it was injected during the prepubertal period from 5 to 20 days no significant influence of MT was observed [5]. In the present study, MT injection for 5 or 10 days or till descent of testes, advanced the age on descent of testes indicating earlier onset of puberty when compared to control animals. The difference in the results of the present study compared to the other studies may be due to the difference in the age of the rats and also duration of MT treatment employed. Further, as the age of the rat advances during the treatment regimen there may be probable shift in the exerting action of MT. MT may bring about this progonadal effect during the juvenile period of male rats by altering the serum gonadotropins, testosterone and growth hormone levels. This finding correlates with the views of Moreno, who stated that neonatal MT administration induces an earlier sexual maturation in male rats [14].
The classical studies of Kennedy and Mitra [15] established that the weight and composition of body is well associated with the timing of sexual maturation. In the present study MT increased the body weight significantly, indicating the rapid general growth than control animals. This may trigger the hypothalamus and prepare for the task of reproduction which is done by the faster growth of the organs in toto. Reverse of this was observed as decreased body weight in the middle aged rats after MT treatment [16] and this difference may be due to variation in the age group as well as the dose of the MT administered. Furthermore, reports regarding the potential role of MT in regulating body weight and metabolism in the young rats are contradictory [17].
Increased testes weight was observed after shorter duration of MT administration in both age rats, while for till descent in 5-day-old rats, and 10 days or till descent in 10-day-old rats does not increase the testes weight. This indicates that within 20 days of postnatal age MT increases testes weight but after that period it does not, may be because of different site of action of MT as the age advances. Moreover, the present results of non-significant decrease in testes weight correlate with the inhibitory action on sexual maturation, when MT was given daily from 20 to 40 days of age by Lang et al. [5]. But the observed non-significant decrease is not sufficient to influence the whole system as far as the onset of puberty is concerned which is indicated by the earlier advancement of age on descent of testes. The similar effect on the epididymides weight in younger rats after MT was observed and this may be due to the direct action by MT on this.
The weight of the thymus gland recorded an increase in all MT treated experimental group in comparison to controls. Experimental evidence reveals that MT is of major importance to thymus development [18]. Long term administration of MT restored the thymus weight which was decreased by pinealectomy [19]. The increased thymus further may exert stimulatory effect on hypothalamus and results in the progonadal effect.
Increased pancreas weight in MT treated animals throws an importance to understand the action of MT on the reproductive axis. This may be one of the factors responsible for the earlier attainment of optimum growth. The results of the present study confirm the reports of presence of MT receptors in pancreas, its stimulatory effect on the functioning of the pancreas [2021]. The increased pancreas weight provides an indirect evidence for the increased IGF concentration. This is hypothesized with the reports of increased levels of IGF after exogenous MT administration [22]. IGFs have been implicated in general growth, definite role in pancreas development. IGF-1 signaling can bring about antiapoptosis, protein synthesis, cell growth and mitogenesis [23]. These properties of IGF are definitely the reason behind the increased weight of pancreas. The present result leads to the hypothesis that MT probably exerts its action on the reproductive axis via IGF and pancreas. But this needs further study to establish whether MT acts on IGF for the growth and development of pancreas, reproductive axis or its stimulatory action on pancreas as a link on the reproductive axis through IGF (Fig. 6). This is the most important presumption leading to the future scope of the present study. IGF-1 is considered as a critical link between the reproductive and somatotropic neuroendocrine systems [24].
The increased testicular weight is supported by increased tubule diameter which was noticed in MT treated groups for shorter duration and thus exerting progonadal influence. Edmonds and Stretson also reported that MT is intimately involved in testicular growth in rice rats [25]. The increased tubule diameter of the testes in the treated rats is an indicative of the action of treatment on higher centers especially on the pituitary gland which through its secretions brings about the changes in the histomorphometric aspects of testes which further accounts for the gross changes. The action at the organ level cannot be ruled out with the fact that testicular morphometry depends on the local secretions also. Increased seminiferous tubule volume and decreased intertubular connective tissue volume in corresponding groups supported the increased testes weight in different treatment combination groups. Surprisingly no change was found in both the age group rats treated with MT till descent reflecting the non-significant decrease in gross testicular weight.
The volume fraction of in-situ thymus gland cortex was significantly increased and medullary volume fraction was decreased in MT treated rats when compared to control rats. These changes possibly reflect the increased weight of in-situ thymus. This agrees with the work which reports the immunostimulatory effect of MT acting on the thymic lymphocytes and epithelial cells by increasing the cellularity of the thymus and hypertrophy after constant darkness [26]. Increase in thymus weight in the present study was supported by increased cortical volume. The increased cortical volume fraction observed in the present study may account for the increased activity of the cortical cells, thereby increasing the secretions of the same which may be responsible for bringing about the progonadal action by acting at the higher centers as well as at the organ level. However, MT acting on its own to increase cortical volume fraction is confirming the report of Herradon et al. [18], that MT is of major importance to thymus development. The results of cortico-medullary volume fraction ratio account for the increased thymus weight in the respective treatment groups. MT treatment in younger rats resulted in higher cortical area, the same was observed in 10-day-old rats treated for 5-day duration which indicates that age and the duration of the treatment are vital for cortical area.
A striking feature of the effects of MT on LH and follicle-stimulating hormone (FSH) release is that they are lost during the course of development [27]. The faster maturation of testes may exert stimulatory role on epididymides microstructure and these in turn may account for the observed gross changes leading to the progonadal state.
Serum levels of gonandotropins were found decreased in adult rats after treatment of MT subcutaneously for 14 days [6]. MT injected daily for 15 or 20 days to rats of age 20 up to 40 days resulted in marked decrease in serum LH concentrations [28]. The results of the present study indicate the increased serum LH levels in both age groups of rats of different duration except in rats treated till descent. The deviatory results of the present study to the other workers may be due to the difference in the age of the rats and also duration of treatment compared to the earlier studies. The non-significant rise in longer duration treated animals of the present study correlate with the report that the effects of MT on LH and FSH release are being ineffective during the course of development [27]. The present finding is in line with that of Esquifino et al., who reported that exposure to MT early in life may accelerate maturation of 24-hour prolactin and LH profiles toward an adult form [29]. The increased LH concentrations may probably due to the MT modulation of gonadotropin secretion by acting on a dopamine mechanism or by acting on the hypothalamus. MT can modulate gonadotropin secretion by acting on a dopamine mechanism independent of hypothalamic suprachiasmatic areas [30].
MT acts not only at higher centres to influence serum hormone levels but also at organ level; this fact was confirmed by the findings of serum testosterone concentrations. This conclusion can be drawn from the significant increase in testosterone concentrations after treatment with MT for different durations. MT binding sites have been characterized in the rat testes, additionally the presence of binding sites in immature rat testes; have been linked for a possible direct role of MT on testicular steroidogenesis [3132]. These reports support the action of MT at organ level i.e., testes which is the site of production of testosterone. Additionally, action of MT at higher centres which brings about increased LH levels which is responsible for the increased serum testosterone concentrations. However, the present findings differ from that of Mandal et al. [6], Olivares et al. [28], probably due to difference in the age of the animals and also duration of treatment employed. The increased testosterone concentrations in experimental animals may be responsible for the faster growth of the reproductive organs directing towards the earlier maturation which probably has resulted in the earlier onset of puberty by advancing the age on descent of testes.
Growth hormone levels were not altered by MT [34]. However, the acute oral administration of MT was found to increase growth hormone levels [35]. The response of growth hormone to growth hormone releasing hormone has been shown to increase after MT treatment [36]. In the present study also MT increased GH concentrations in all the groups. The GH concentrations may probably result in the increased secretion of IGF-1. This is hypothesized with the background of the report stating that the deficiency of GH results in a low state of IGF-1 thereby affecting translational efficiency and secretory responses of both the hormones [33]. This is further substantiated with increase in pancreas weight which may also contribute for increased IGF-1 in turn for the progonadal action of MT. The increase in GH levels seen in the present series may be connected to the increase in the body weight and other histological parameters of reproductive organs concerned which stated to be associated with the sexual maturity as described by Kennedy and Mitra [15] accounting for the progonadal form of action as a possible mechanism for early descent of testes (Fig. 7).
References
1. Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem. 1960; 235:1992–1997. PMID: 14415935.
2. Das UN. Melatonin in human health and disease. J Assoc Phys India. 1996; 45:133–139.
3. Weaver DR. The roles of melatonin in development. In : Olcese J, editor. Melatonin after Four Decades. New York: Kluwer Academic/Plenum Publishers;2000. p. 199–214.
4. Minneman KP, Wurtman RJ. Effects of pineal compounds on mammals. Life Sci. 1975; 17:1189–1199. PMID: 1105043.
5. Lang U, Aubert ML, Conne BS, Bradtke JC, Sizonenko PC. Influence of exogenous melatonin on melatonin secretion and the neuroendocrine reproductive axis of intact male rats during sexual maturation. Endocrinology. 1983; 112:1578–1584. PMID: 6299701.
6. Mandal H, Ghosh PK, Biswas NM. Effect of dihydrotestosterone on serum concentrations of alpha 2u-globulin and on spermatogenesis in melatonin-treated rats. J Endocrinol. 1990; 126:431–435. PMID: 1698906.
7. Pierpaoli W, Bulian D, Dall'Ara A, Marchetti B, Gallo F, Morale MC, Tirolo C, Testa N. Circadian melatonin and young-to-old pineal grafting postpone aging and maintain juvenile conditions of reproductive functions in mice and rats. Exp Gerontol. 1997; 32:587–602. PMID: 9315459.
8. Alonso-Solís R, Abreu P, López-Coviella I, Hernández G, Fajardo N, Hernández-Díaz F, Díaz-Cruz A, Hernández A. Gonadal steroid modulation of neuroendocrine transduction: a transynaptic view. Cell Mol Neurobiol. 1996; 16:357–382. PMID: 8818402.
9. Yilmaz B, Kutlu S, Mogulkoç R, Canpolat S, Sandal S, Tarakçi B, Kelestimur H. Melatonin inhibits testosterone secretion by acting at hypothalamo-pituitary-gonadal axis in the rat. Neuro Endocrinol Lett. 2000; 21:301–306. PMID: 11455362.
10. Relkin R. Absence of alteration in puberal onset in male rats following amygdaloid lesioning. Endocrinology. 1971; 88:1272–1274. PMID: 5547247.
11. Farris EJ, Griffith JQ Jr. The rat: in laboratory investigation. 2nd ed. Oxford: J.B.Lippincott Co.;1949.
12. Drury RA, Wallington EA. Carleton's histological technique. 4th ed. New York: Oxford University Press;1967.
13. Rat Behavior and Biology (Anne's rat page). Biological statistics of the Norway rat [Internet]. Rat Behavior and Biology;2003, 2004. cited 2019 Jan 1. Available from: http://www.ratbehavior.org/Stats.htm#RatStats.
14. Moreno ML, Villanua MA, Esquifino AI. Serum prolactin and luteinizing hormone levels and the activities of hypothalamic monoamine oxidase A and B and phenylethanolamine-N-methyl transferase are changed during sexual maturation in male rats treated neonatally with melatonin. J Pineal Res. 1992; 13:167–173. PMID: 1287192.
15. Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963; 166:408–418. PMID: 14031944.
16. Rasmussen DD, Boldt BM, Wilkinson CW, Yellon SM, Matsumoto AM. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999; 140:1009–1012. PMID: 9927336.
17. Peschke D, Peschke E, Mess B. Circannual rhythm and increase of body weight and food intake in the young wistar rat following pinealectomy and ganglionectomy. Neuroendocrinol Lett. 1987; 9:321–327.
18. Herradon PG, Razquin B, Zapata AG. Effects of early partial decapitation on the ontogenic development of chicken lymphoid organs. I. Thymus. Am J Anat. 1991; 191:57–66. PMID: 1648305.
19. Oner H, Kus I, Oner J, Ogeturk M, Ozan E, Ayar A. Possible effects of melatonin on thymus gland after pinealectomy in rats. Neuro Endocrinol Lett. 2004; 25:115–118. PMID: 15159694.
20. Leja-Szpak A, Jaworek J, Nawrot-Porabka K, Palonek M, Mitis-Musioł M, Dembiński A, Konturek SJ, Pawlik WW. Modulation of pancreatic enzyme secretion by melatonin and its precursor: L-tryptophan. Role of CCK and afferent nerves. J Physiol Pharmacol. 2004; 55 Suppl 2:33–46. PMID: 15608359.
21. Jaworek J, Nawrot K, Konturek SJ, Leja-Szpak A, Thor P, Pawlik WW. Melatonin and its precursor, L-tryptophan: influence on pancreatic amylase secretion in vivo and in vitro. J Pineal Res. 2004; 36:155–164. PMID: 15009505.
22. Ostrowska Z, Kos-Kudla B, Swietochowska E, Marek B, Kajdaniuk D, Ciesielska-Kopacz N. Influence of pinealectomy and long-term melatonin administration on GH-IGF-I axis function in male rats. Neuro Endocrinol Lett. 2001; 22:255–262. PMID: 11524633.
23. van Haeften TW, Twickler TB. Insulin-like growth factors and pancreas beta cells. Eur J Clin Invest. 2004; 34:249–255. PMID: 15086355.
24. Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med (Maywood). 2005; 230:292–306. PMID: 15855296.
25. Edmonds KE, Stetson MH. Photoperiod and melatonin affect testicular growth in the marsh rice rat (Oryzomys palustris). J Pineal Res. 1994; 17:86–93. PMID: 7869231.
26. Mahmoud I, Salman SS, al-Khateeb A. Continuous darkness and continuous light induce structural changes in the rat thymus. J Anat. 1994; 185(Pt 1):143–149. PMID: 7559109.
27. Vaněcek J. The melatonin receptors in rat ontogenesis. Neuroendocrinology. 1988; 48:201–203. PMID: 2851752.
28. Olivares AN, Valladares LE, Bustos-Obregón E, Núñez SM. Testicular function of sexually immature rats chronically treated with melatonin. Arch Biol Med Exp (Santiago). 1989; 22:387–393. PMID: 2488537.
29. Esquifino AI, Arce A, Villanúa MA, Cardinali DP. Development of 24-hour rhythms in serum prolactin and luteinizing hormone levels in rats neonatally administered melatonin. Chronobiol Int. 1998; 15:21–28. PMID: 9493711.
30. Acuña-Castroviejo D, Fernández B, Castillo JL, del Aguila CM. Similarity between the effects of suprachiasmatic nuclei lesions and of pinealectomy on gonadotropin release in ovariectomized, sulpiride-treated and melatonin-replaced rats. Experientia. 1993; 49:797–801. PMID: 8405305.
31. Tijmes M, Pedraza R, Valladares L. Melatonin in the rat testis: evidence for local synthesis. Steroids. 1996; 61:65–68. PMID: 8750434.
32. Vera H, Tijmes M, Valladares LE. Melatonin and testicular function: characterization of binding sites for 2-[125I]-iodomelatonin in immature rat testes. Steroids. 1997; 62:226–229. PMID: 9055381.
33. Jevdjovic T, Maake C, Zwimpfer C, Krey G, Eppler E, Zapf J, Reinecke M. The effect of hypophysectomy on pancreatic islet hormone and insulin-like growth factor I content and mRNA expression in rat. Histochem Cell Biol. 2005; 123:179–188. PMID: 15812646.
34. Kostoglou-Athanassiou I, Treacher DF, Wheeler MJ, Forsling ML. Melatonin administration and pituitary hormone secretion. Clin Endocrinol (Oxf). 1998; 48:31–37. PMID: 9509065.
35. Smythe GA, Lazarus L. Growth hormone responses to melatonin in man. Science. 1974; 184:1373–1374. PMID: 4833260.
36. Valcavi R, Dieguez C, Azzarito C, Edwards CA, Dotti C, Page MD, Portioli I, Scanlon MF. Effect of oral administration of melatonin on GH responses to GRF 1-44 in normal subjects. Clin Endocrinol (Oxf). 1987; 26:453–458. PMID: 3115632.
Fig. 1
(A) Control rat testis (H&E stain, ×100) showing normal tubular diameter, less compactness of tubules and normal interstitial connective tissue. (B) Melatonin (MT) treated rat testis showing increased tubular diameter, more compactness of tubules and reduced interstitial connective tissue (H&E stain, ×100). (C) Control rat testis (H&E stain, ×500) showing normal tubular diameter, less compactness of tubules and normal interstitial connective tissue. (D) MT treated rat testis showing increased tubular diameter, more compactness of tubules and reduced interstitial connective tissue (H&E stain, ×500).
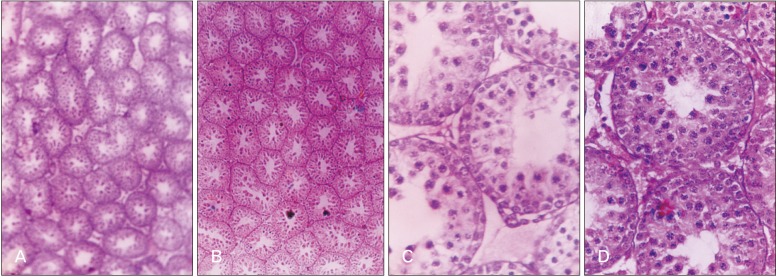
Fig. 2
(A) In situ thymus gland of control rats (H&E stain, ×125) showing normal central medulla and peripheral cortex. (B) In situ thymus gland of melatonin treated rats showing increased, dense, wider cortex and lesser medulla (H&E stain, ×125).
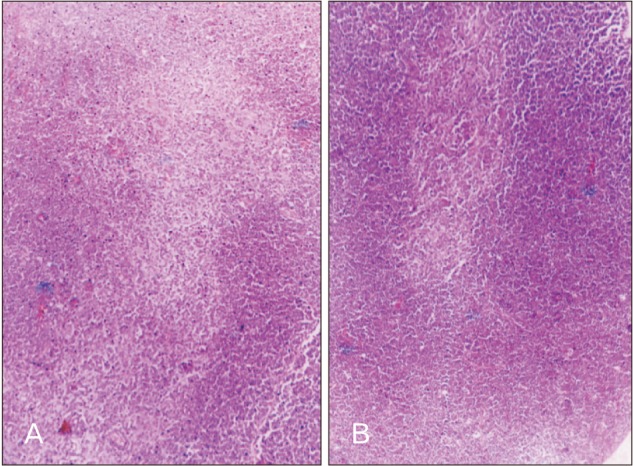
Fig. 3
(A) Effect of melatonin (MT) on luteinizing hormone (LH) in 5-day-old rats. MT treatment shows significant increase in LH levels compared to control. (B) Effect of MT on LH in 10-day-old rats. MT treatment shows significant increase in LH levels compared to control. *P<0.05, **P<0.01.
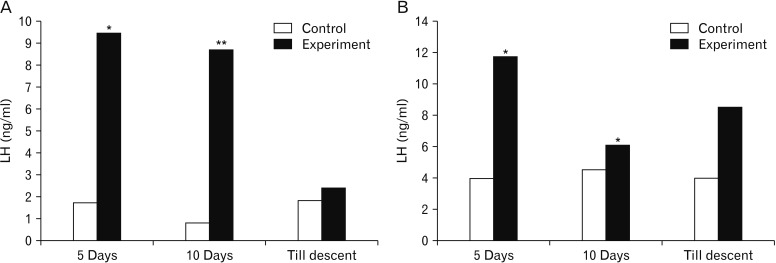
Fig. 4
(A) Effect of melatonin (MT) on testosterone in 5-day-old rats. MT treatment shows significant increase in testosterone levels compared to control. (B) Effect of MT on testosterone in 10-day-old rats. MT treatment shows significant increase in testosterone levels compared to control. *P<0.05, **P<0.01.
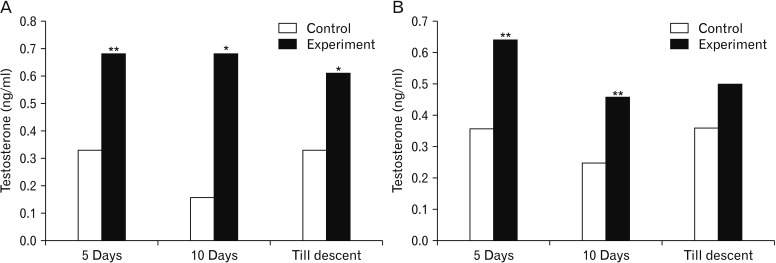
Fig. 5
(A) Effect of melatonin (MT) on growth hormone (GH) in 5-day-old rats. MT treatment shows significant increase in GH levels compared to control. (B) Effect of MT on GH in 10-day-old rats. MT treatment shows significant increase in GH levels compared to control. *P<0.05, **P<0.01.
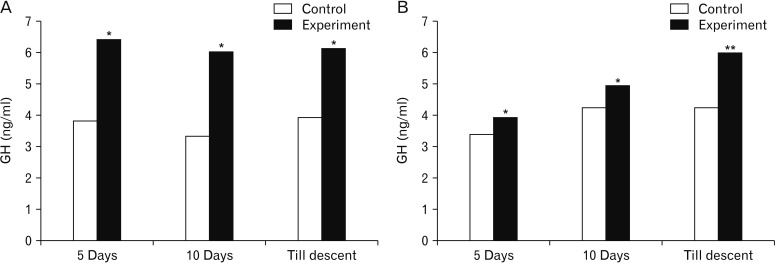
Fig. 6
Schematic representation of action of melatonin on pancreas. IGF, insulin-like growth factor.
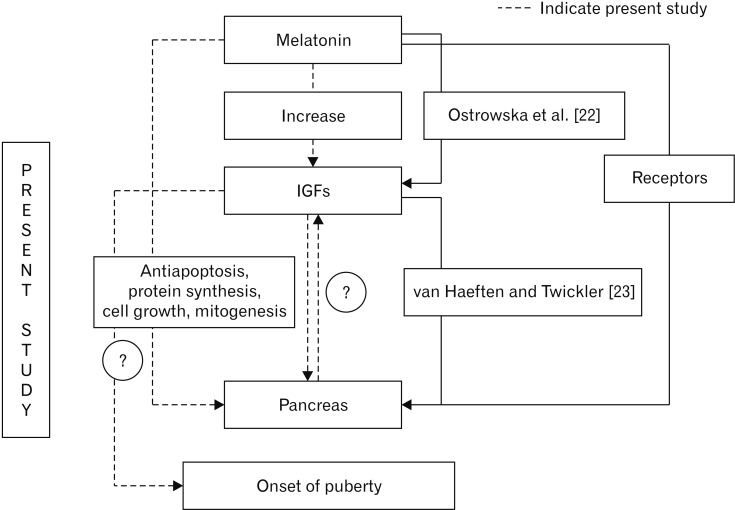
Fig. 7
Schematic representation of mechanism of action of melatonin on gross, histological and hormonal parameters. LH, luteinizing hormone; GH, growth hormone; IGF-1, insulin-like growth factor 1.
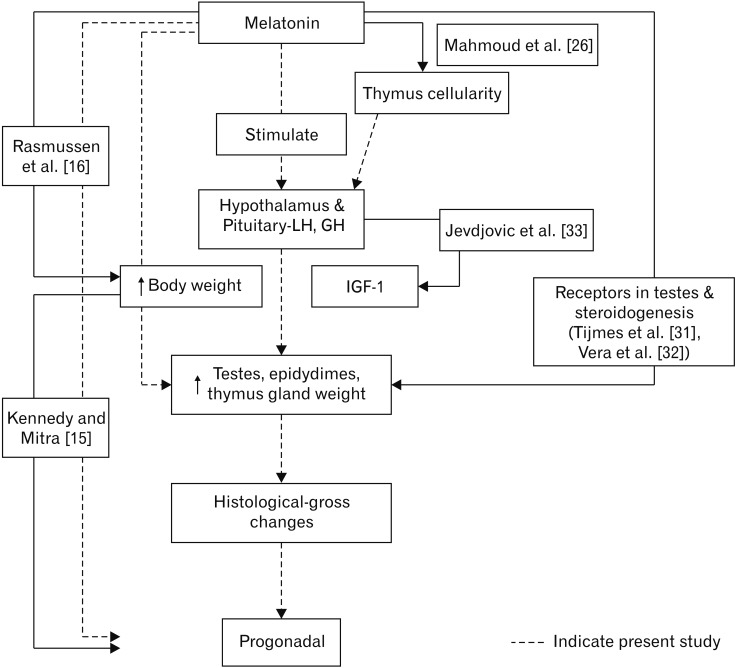
Table 1
Effect of melatonin on descent of testes and body, testes, epidydimides, thymus and pancreas weight in 5-day-old rats
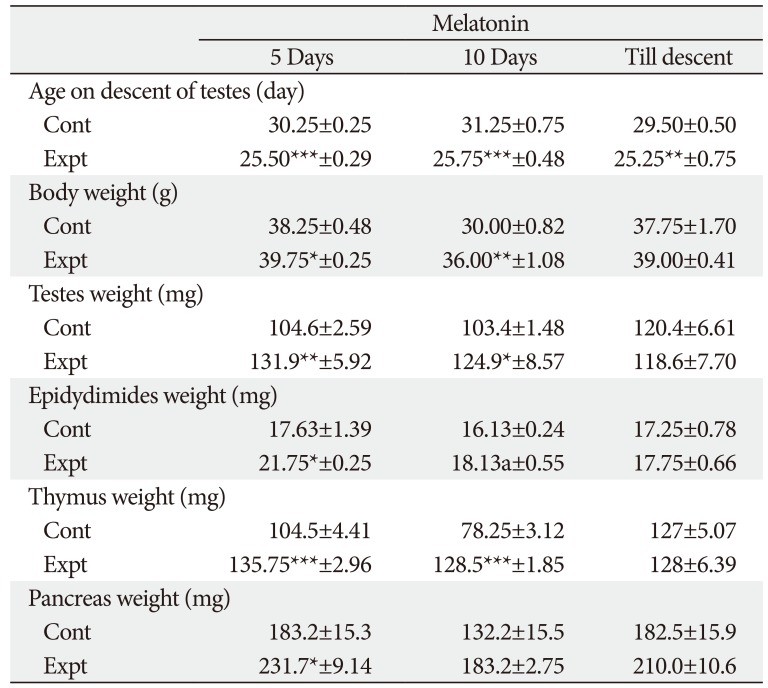
Table 2
Effect of melatonin on descent of testes and body, testes, epidydimides, thymus and pancreas weight in 10-day-old rats
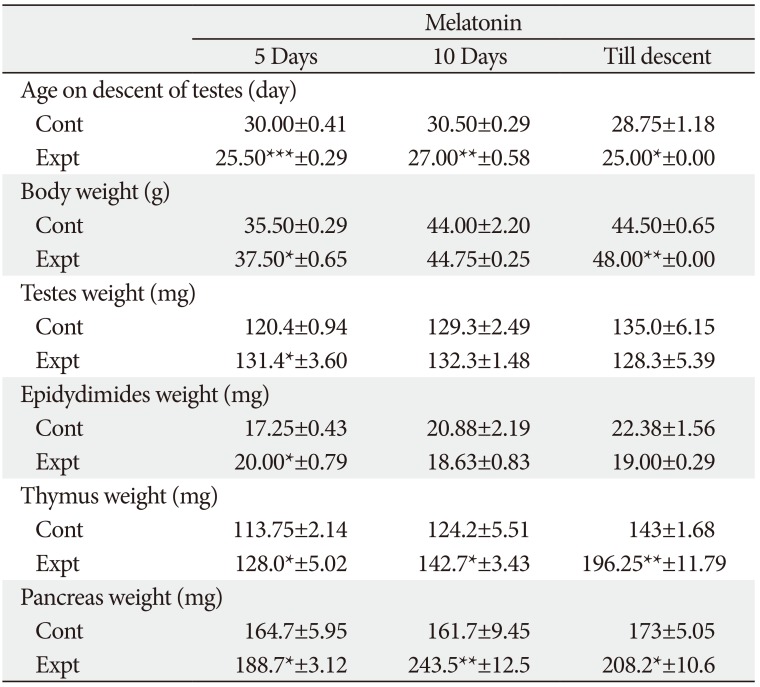
Table 3
Effect of melatonin on histomorphometry of testis in 5-day-old and 10-day-old rats
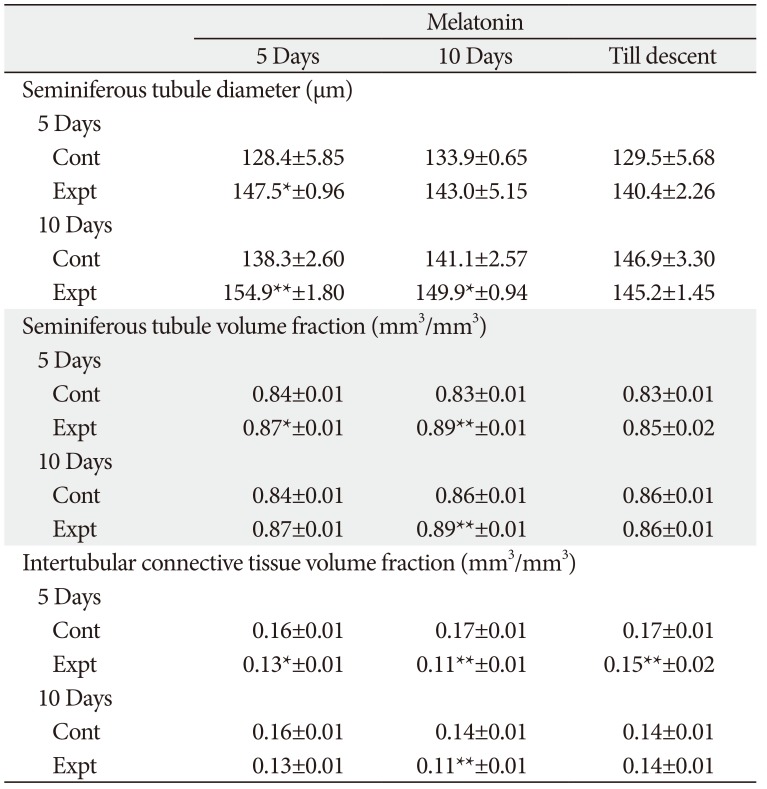
Table 4
Effect of melatonin on histomorphometry of thymus in 5-day-old and 10-day-old rats




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download