Abstract
The human nervus terminalis (terminal nerve) and the nerves to the vomeronasal organ (VNON) are both associated with the olfactory nerves and are of major interest to embryologists. However, there is still limited knowledge on their topographical anatomy in the nasal septum and on the number and distribution of ganglion cells along and near the cribriform plate of the ethmoid bone. We observed serial or semiserial sections of 30 fetuses at 7–18 weeks (crown rump length [CRL], 25–160 mm). Calretinin and S100 protein staining demonstrated not only the terminal nerve along the anterior edge of the perpendicular lamina of the ethmoid, but also the VNON along the posterior edge of the lamina. The terminal nerve was composed of 1–2 nerve bundles that passed through the anterior end of the cribriform plate, whereas the VNON consisted of 2–3 bundles behind the olfactory nerves. The terminal nerve ran along and crossed the posterior side of the nasal branch of the anterior ethmoidal nerve. Multiple clusters of small ganglion cells were found on the lateral surfaces of the ethmoid's crista galli, which are likely the origin of both the terminal nerve and VNON. The ganglions along the crista galli were ball-like and 15–20 µm in diameter and, ranged from 40–153 in unilateral number according to our counting at 21-µm-interval except for one specimen (480 neurons; CRL, 137 mm). An effect of nerve degeneration with increasing age seemed to be masked by a remarkable individual difference.
The human nervus terminalis (terminal nerve) and the nerves to the vomeronasal organ (VNON) are both associated with the olfactory nerves and have been of major interest to embryologists. The VNON was termed Organum vomeronasale or Jacobson's organ. Using mammalian fetuses, including those of humans, previous studies provided beautiful line drawings of these nerves and the associated ganglion at the anterior skull base [1234]. Some researchers pointed out to the presence of these nerves even in human adults [567]. Müller and O'rahilly [8] reported that (1) the VNON makes a bundle separated from the olfactory nerves and terminal nerve and (2) the terminal nerve crosses the deep or posterior side of the anterior ethmoidal nerve with or without communication. Pearson [4] also described anastomosis between the terminal nerve and the anterior ethmoidal nerve in a fetus at a crown rump length (CRL) of 45 mm.
However, according to our interpretations, previous studies failed to obtain (1) photos at low magnification showing the topographical anatomy of these nerves in the nasal septum and (2) the distribution patterns of the ganglion cells at the cribriform plate of the ethmoid bone. Therefore, to provide a better understanding of the anatomy, the first aim of this study was to demonstrate photographically both the terminal nerve and VNON including their ganglion cells in human fetuses. According to Smith and Bhatnagar [9], the human VNON increases in size from 32 days to 28 weeks of gestation. Moreover, the VNON is found in 23% of adult patients [10]. Thus, the degeneration of the associated ganglion cells is likely to start after birth. Likewise, the terminal nerve is also found in adults [1356], although most of those reports were based on macroscopic observations. The second aim of this study was to evaluate histologically the number of ganglion cells as a function of the age of human fetuses.
Gonadotropin releasing hormones [71112] and calcium-binding proteins such as calretinin [1314151617] are considered good markers for both the terminal nerve and VNON. In contrast to gonadotropin releasing hormones, immunoreactivity of calcium-binding proteins is well preserved even in formalin-fixed, long-preserved fetuses [18]. Thus, we used calretinin as a specific marker in addition to the S100 protein as a general marker of peripheral nerves. S100-negatiivity of ganglion cell bodies were consistent with cardiac ganglion cells in human fetuses of the similar age [1920].
This study was performed in accordance with the principles of the Declaration of Helsinki 1995 (as revised in 2013). We observed semiserial sagittal sections of the head of 10 fetuses at 7–18 weeks (CRL, 27–160 mm) and serial frontal sections of 20 fetuses of the same age (CRL, 25–150 mm). The sagittal specimens were obtained in China, whereas the frontal specimens were obtained in Spain.
The 10 sagittal specimens were donated by the fetuses' families to the Department of Anatomy, Yanbian University Medical College, Yanji, China, until 2016 and their use for research was approved by the university ethics committee in Yanji (No. BS-13-35). These fetuses were obtained by induced abortion, after which the mother was orally informed by an obstetrician at the college teaching hospital of the possibility of donating the fetus for research; no attempt was made to encourage the donation actively. After the mother agreed, the fetus was assigned a specimen number and stored in 10% w/w neutral formalin solution for more than 1 month. Because of specimen number randomization, there was no possibility of contacting the family at a later date. After dividing the body into parts, head samples were decalcified by incubating at 4℃ in a 0.5-mol/l EDTA (pH 7.5) decalcifying solution (Decalcifying Solution B, Wako, Tokyo, Japan) for 3–5 days, depending on the size of the sample. The head specimens were sectioned sagittally at 20–50-µm intervals depending on sizes of the materials and were cut at a 5-µm thickness.
A minor part of sagittal sections from six of the 10 Chinese specimens were used for immunohistochemistry (CRL: 35 mm, 45 mm, 60 mm, 85 mm, 110 mm, and 160 mm), whereas the residual, major part of sections were used for hematoxylin and eosin (H&E) staining. The primary antibodies used were rabbit monoclonal anti-human calretinin (Invitorogene PA1-31163, Thermo Fisher Scientific, Yokohama, Japan), rabbit monoclonal anti-human S100 (1:100, Dako N1573, Dako, Glostrup, Denmark), or mouse monoclonal anti-human neuron-specific enolase (NSE; 1:200, Dako N1557, Dako). Samples were not pretreated in the autoclave because of the fragile nature of the fetal tissues. Following incubation with the primary antibody, sections were incubated with horseradish peroxidase-labeled secondary antibody (Histofine Simple Stain Max-PO, Nichirei, Tokyo, Japan) for 30 minutes, followed by incubation with diaminobenzidine (Histofine Simple Stain DAB, Nichirei) for 3–5 minutes. All samples were counterstained with hematoxylin.
The 20 Spanish specimens belonged to a large collection kept at the Embryology Institute of the Universidad Complutense, Madrid, and were the products of miscarriages and ectopic pregnancies at the university's Department of Obstetrics. Specimens were cut frontally at 7 µm. Most sections were stained with H&E, while a minor part with azan, orange G, or silver staining. However, we used only sections stained with H&E. The study was approved by the Complutense University ethics committee (B08/374). The observations and photograph acquisitions were performed with Nikon Eclipse 80 (Tokyo, Japan). With manual works performed by two of the authors (K.H.C. and G.M.), the ganglion cells along the crista galli were quantified in every three sections of 16 specimens (i.e., at 21-µm interval) under a ×20 objective lens.
Immunohistochemistry of calretinin and S100 protein clearly demonstrated not only the terminal nerve along the anterior edge of the perpendicular lamina of the ethmoid, but also the VNON along the posterior edge of the lamina. The terminal nerve was composed of 1–2 nerve bundles that passed through the anterior end of the cribriform plate, whereas the VNON consisted of 2–3 bundles behind the olfactory nerves (Figs. 1, 2). In the initial stage, the terminal nerve and VNON were closely located, but they became separated on growth of the nasal septum and cribriform plate as well as on outgrowth of olfactory nerves. Thus, a bundle of the terminal nerve was located 2–3 mm anterior to the VNON in Fig. 2A (15 weeks) in contrast to the two nerves those were closely and parallel located in Fig. 1A (9 weeks). Abundant olfactory nerves became located between the terminal nerve and VNON. The VNON inferior course changed to the relatively horizontal course at its distal parts near the VNON. Calretinin-positive cells were also observed scattered in the nasal epithelium at the cribriform plate and on the upper part of the nasal septum as well as the VNON (Fig. 1B, C).
The anterior ethmoidal nerve ran medially from the orbit and reached the superolateral angle of the developing nasal cavity, and the nasal branch took an inferior course (Figs. 3, 4). In the immediate superior side of the cartilaginous nasal bone, it crossed in the immediately superficial or anterior side of the terminal nerve. Depending on the growth of the nasal bone in thickness, both nerve courses became separated; the anterior ethmoidal nerve became superficial to the bone, while the terminal nerve was located deep to the bone. The ethmoidal nerve appeared pale following H&E staining possibly because of myelination (Fig. 4E).
In the frontal sections of specimens with a CRL larger than 48 mm (later than 10 weeks; 16 of 20 Spanish specimens), we found multiple clusters of small ganglion cells on the lateral surfaces of the ethmoid's crista galli (Fig. 5). These were near the terminal nerve, but likely to issue not only the terminal nerve but also the VNON since the posterior part of the cribriform plate did not carry ganglion cells. The cluster was irregularly shaped and continued through 6–15 sections along the crista galli (42–105 µm; mean, 85 µm) (Table 1). The ganglions were ball-like with a 15–20 µm diameter and contained a large nucleus. They were positive for NSE and calretinin, negative for S100, and their cytoplasm was highly eosinophilic (Fig. 5C, E, F). In contrast, these neurons were absent along the olfactory nerves at the cribriform plate and nasal septum. Thus, the round ganglion cells were unlikely to be usual olfactory neurons. Likewise, we did not find such neurons at and near the connections of the nerves to the forebrain. However, visualization of the subarachnoid space was not feasible in almost half of the preset specimens because of damage during the histological procedures. Because of their small size (<20 µm), we estimated the ganglion cell number by quantifying them in every three sections by using a ×20 objective. Unilaterally, the number of ganglion cells along the crista galli ranged from 40 to 153 (mean, 65) (Table 1) except for a much greater number found in one specimen (480 neurons; CRL 137 mm).
The most striking observation in the present study was the visualization of ganglion cell clusters on the lateral surfaces of the crista galli of the ethmoid. In previous reports on human fetuses, all or most of the ganglion cells were distributed at and near the connections of the nerves to the forebrain. Larsell [2] reported multiple ganglion cells along the nerve near the brain in human fetuses. Similarly, in his line-drawings of 19 human embryos and fetuses (CRL, 9–78 mm), Pearson [4] reported small groups of bipolar and multipolar ganglion cells along the proximal most part of the terminal nerve.
Although we did not observe any ganglion cells at the proximal nerve course near the brain, we assume that this may be due to the bad condition of the subarachnoid space in many of the present specimens. Therefore, our counting of ganglion cells seemed to be underestimated. Patron et al. [21] presented a limited description of human ganglion cells on the crista galli and considered the neurons as the terminal nerve. Pearson [4] discriminated his terminalis nerve ganglion from ganglion cells of the VNON by using silver impregnation; the terminal nerve was stained much more densely than the VNON by his method. However, we were not able to determine whether the ganglion cell clusters were of the terminal nerve or the VNON. We assumed that they might include cell bodies of both nerves.
According to a table provided by Müller and O'rahilly [8], the ganglion cells of the terminal nerve and VNON appeared at 6 weeks. However, the authors did not provide any photographic evidence. In our study, the ganglion cells on the crista galli were detected in specimens larger than 48 mm CRL. We counted the numbers of ganglion cell bodies according to observations of one of three sections (at 21-µm interval). It ranged from 40–153 in unilateral number according to our counting at 21-µm interval except for one specimen (480 neurons; CRL, 137 mm). However, because of underestimation due to smaller ganglion cell bodies mixed, we roughly estimated at 50–200 ganglion cell bodies per one side of the crista galli. Notably, the number of ganglion cells as well as the size of ganglion cell cluster did not clearly decrease with increasing age of the fetuses (Table 1). The greater number was found not only in large fetuses (CRL, 137 mm and 115 mm) but in small ones (CRL, 48 mm). Thus, an effect of nerve degeneration seemed to be masked by individual difference. This fact seemed to be consistent with an increased size of the human VNON during the fetal stages [9], in addition to the persistent presence of the terminal nerve in adults [567]. However, we did not deny a possibility that neurons nearby brain start degeneration earlier than those on the crista galli.
There seemed to be few quantitative studies of the vomeronasal nerve-associated ganglion cells bodies, i.e., paravomeronasal ganglia [9], in a field of the comparative anatomy. A limited example was found in Bhatnagar and Kallen [22] who counted paravomeronasal ganglion cell bodies in insectivorous bats. According to them, of the 396 cell bodies per animal, ten were found in the nerve a short distance posterior to the VNON and 28 were found at random depths within the epithelium of the organ. Thus, the residual 358 neurons seemed to be most likely analogue of the present human ganglia along the crista galli. Therefore, in number, human neurons might be 10% of those of bats when compared with the present data. However, in rabbit fetuses, a number of ganglion cells on the crista galli seemed to contain almost 10% of terminalis nerve neurons [23]. Recently, Peňa-Melian et al. (2019) [24] described the VNON and terminal nerve containing ganglion cell bodies along the crista galli in a human fetus of 76 mm CRL, but we were difficult to identify them in their lower magnification photo.
Our immunohistochemistry findings demonstrated a topographical anatomy of the terminal nerve and VNON in the nasal septum, whereby the former ran along the anterior edge of the perpendicular lamina, whereas the latter ran along the posterior edge. Previous studies offered no clear description of the fact that the olfactory nerves interposed between the terminal nerve and VNON at the cribriform plate in midterm fetuses. A separation process of the VNON from the olfactory nerves seemed to depend on the growth in anteroposterior length of the cribriform plate. In either of the terminal nerve and VNON, there were less than four nerve bundles. These were much smaller than those described in illustrations by McCotter [3] and Brookover [5], but were almost consistent with those illustrated by Pearson [4]. These previous discrepancies may be the result of over-emphasis that is likely to occur in studies based on macroscopic observations. Finally, the cribriform plate is known as a focus of cerebrospinal fluid fistula to cause pathological rhinorrhea [25].
References
1. Johnston JB. The nervus terminalis in man and mammals. Anat Rec. 1914; 8:185–198.
2. Larsell O. The nervus terminalis. Ann Otol Rhinol Laryngol. 1950; 59:414–438. PMID: 15426155.
3. McCotter RE. A note on the course and distribution of the nervus terminalis in man. Anat Rec. 1915; 9:243–246.
4. Pearson AA. The development of the nervus terminalis in man. J Comp Neurol. 1941; 75:39–66.
5. Brookover C. The nervus terminalis in adult man. J Comp Neurol. 1914; 24:131–135.
6. Fuller GN, Burger PC. Nervus terminalis (cranial nerve zero) in the adult human. Clin Neuropathol. 1990; 9:279–283. PMID: 2286018.
7. Kim K, Patel L, Tobet S, King J, Rubin B, Stopa E. Gonadotropin-releasing hormone immunoreactivity in the adult and fetal human olfactory system. Brain Res. 1999; 826:220–229. PMID: 10224299.
8. Müller F, O'rahilly R. Olfactory structures in staged human embryos. Cells Tissues Organs. 2004; 178:93–116. PMID: 15604533.
9. Smith TD, Bhatnagar KP. The human vomeronasal organ. Part II: prenatal development. J Anat. 2000; 197:421–436. PMID: 11117628.
10. Besli R, Saylam C, Veral A, Karl B, Ozek C. The existence of the vomeronasal organ in human beings. J Craniofac Surg. 2004; 15:730–735. PMID: 15346008.
11. Demski LS. Terminal nerve complex. Acta Anat (Basel). 1993; 148:81–95. PMID: 8109200.
12. Wirsig-Wiechmann CR, Wiechmann AF, Eisthen HL. What defines the nervus terminalis? Neurochemical, developmental, and anatomical criteria. Prog Brain Res. 2002; 141:45–58. PMID: 12508560.
13. Bédard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res Dev Brain Res. 2004; 151:159–168. PMID: 15246702.
14. Bastianelli E, Polans AS, Hidaka H, Pochet R. Differential distribution of six calcium-binding proteins in the rat olfactory epithelium during postnatal development and adulthood. J Comp Neurol. 1995; 354:395–409. PMID: 7541806.
15. Johnson EW. CaBPs and other immunohistochemical markers of the human vomeronasal system: a comparison with other mammals. Microsc Res Tech. 1998; 41:530–541. PMID: 9712200.
16. Johnson EW. Immunocytochemical characteristics of cells and fibers in the nasal mucosa of young and adult macaques. Anat Rec. 2000; 259:215–228. PMID: 10820323.
17. Malz CR, Knabe W, Kuhn HJ. Calretinin immunoreactivity in the prenatally developing olfactory systems of the tree shrew Tupaia belangeri. Anat Embryol (Berl). 2002; 205:83–97. PMID: 12021911.
18. Katori Y, Jin ZW, Kawase T, Hong KH, Murakami G, Cho BH. Developmental changes in the distribution of calretinin-immunoreactive cells in human fetal nasal epithelium. Okajimas Folia Anat Jpn. 2010; 87:5–10. PMID: 20715566.
19. Kim JH, Cho KH, Jin ZW, Murakami G, Abe H, Chai OH. Ganglion cardiacum or juxtaductal body of human fetuses. Anat Cell Biol. 2018; 51:266–273. PMID: 30637161.
20. Cho KH, Kim JH, Murakami G, Abe H, Rodríguez-Vázquez JF, Chai OH. Nerve distribution in myocardium including the atrial ventricular septa in late stage human fetuses. Anat Cell Biol. 2019; 52:48–56. PMID: 30984452.
21. Patron V, Berkaoui J, Jankowski R, Lechapt-Zalcman E, Moreau S, Hitier M. The forgotten foramina: a study of the anterior cribriform plate. Surg Radiol Anat. 2015; 37:835–840. PMID: 25823692.
22. Bhatnagar KP, Kallen FC. Morphology of the nasal cavities and associated structures in Artibeus jamaicensis and Myotis lucifugus. Am J Anat. 1974; 139:167–189. PMID: 4812217.
23. Huber GC, Guild SR. Observations on the peripheral distribution of the nervus terminalis in mammalia. Anat Rec. 1913; 7:253–272.
24. Peña-Melián Á, Cabello-de la Rosa JP, Gallardo-Alcañiz MJ, Vaamonde-Gamo J, Relea-Calatayud F, González-López L, Villanueva-Anguita P, Flores-Cuadrado A, Saiz-Sánchez D, Martínez-Marcos A. Cranial pair 0: the nervus terminalis. Anat Rec (Hoboken). 2019; 302:394–404. PMID: 29663690.
25. Dare AO, Balos LL, Grand W. Neural-dural transition at the medial anterior cranial base: an anatomical and histological study with clinical applications. J Neurosurg. 2003; 99:362–365. PMID: 12924711.
Fig. 1
The terminal nerve and nerves to the vomeronasal organ (VNON) are closely located in a specimen of crown rump length 45 mm (9 weeks). Sagittal sections. Immunohistochemistry of S100 protein (S100) (A, D, E) or calretinin (cal) (B, C, F, G). Panels A and D display the lateral planes in the figure, while panels B, C, F, and G display the medial planes. Thus, the upper or lower raw of panels exhibits a different side from the lateral to the medial, respectively. Intervals between panels are 0.2 mm (A–B), 0.1 mm (B–C), 0.2 mm (D–E), and 0.1 mm (E–F, F–G). On one side (panels A–C), the proximal course of the VNON was not cut, and we did not identify the distal course of the terminal nerve (NT) on the other side (panels D–G). Open stars in panels E and F indicate the most likely candidate of the terminal nerve. Arrowhead in panel D indicates an on-going fusion between the nasal septum and palate. In the specimen, the terminal nerve and VNON appeared to be closely located in the nasal septum. VNO, vomeronasal organ. Scale bars=1 mm (A–G).
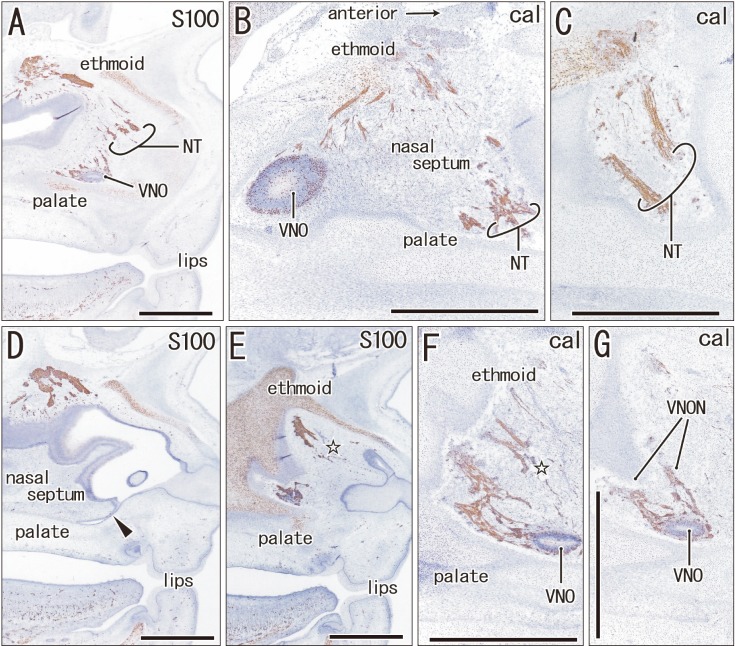
Fig. 2
The terminal nerve and nerves to the vomeronasal organ (VNON) are separated by a developing nasal septum in a specimen of crown rump length 110 mm (15 weeks). Sagittal sections. Immunohistochemistry of S100 protein (S100) (A, C) or calretinin (cal) (D). (B) Hematoxylin and eosin staining. Panels C and D display the vomeronasal organ (VNO) and VNON in the other side of panels A and B. Intervals between panels are 0.2 mm (A–B), 0.6 mm (B–C), and 0.1 mm (C–D). In panel A, the terminal nerve (NT) runs along the posterior aspect of the cartilaginous nasal bone, while the VNON run along the palate near the VNO. Thus, these nerves are separated by a developing nasal septum. Scale bars=1 mm (A–D).
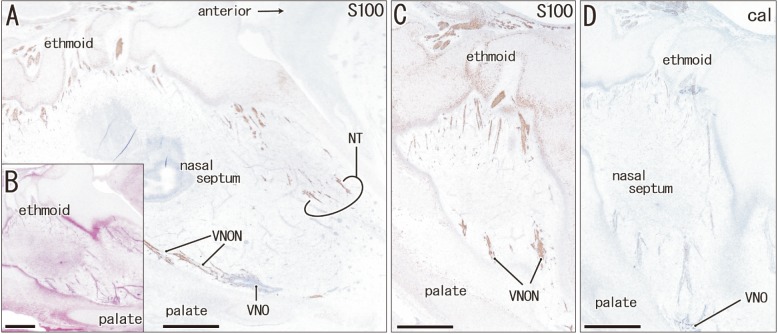
Fig. 3
Anterior ethmoidal nerve coming from the orbit in a specimen of crown rump length 27 mm (7 weeks). Sagittal sections. Hematoxylin and eosin staining. Panel A (or F) displays the most lateral (or medial) plane in the figure. Intervals between panels are 0.3 mm (A–B, B–C), 0.05 mm (C–D, D–E), and 0.1 mm (E–F). The anterior ethmoidal nerve (AEN) runs medially from the orbit (A–C), reaches the superolateral angle of the nasal cavity (D), and directs inferiorly along the mucosal membrane (E, F). Panels A–D were prepared at the same magnification (scale bar in panel A=1 mm). FN, frontal nerve; MC, Meckel's cartilage; OCN, oculomotor nerve; PPG, pterygopalatine ganglion; TG, trigeminal nerve ganglion.
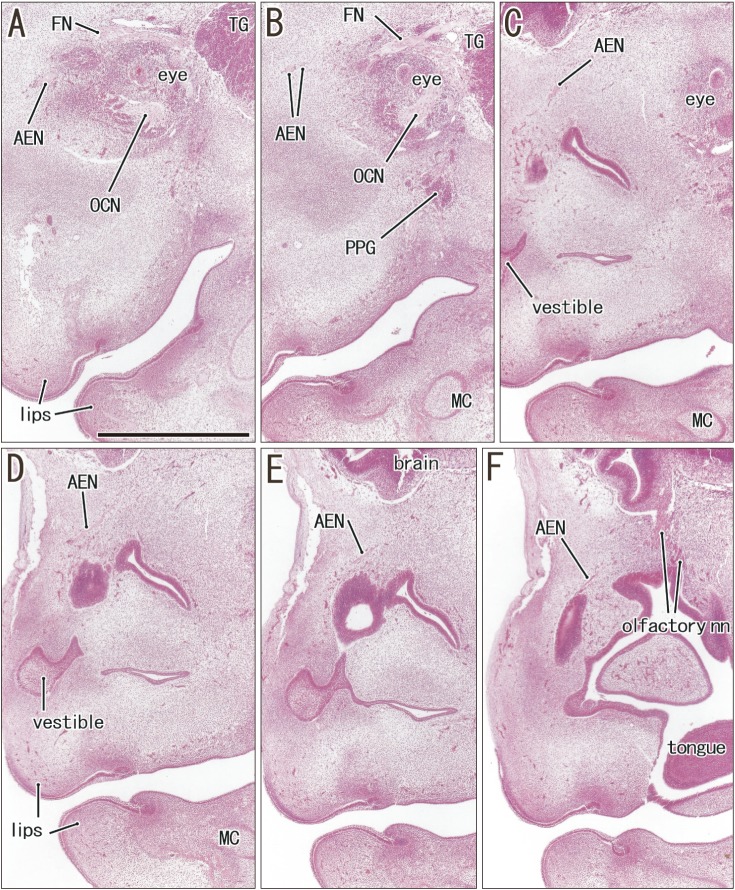
Fig. 4
Anterior ethmoidal nerve crossing superficially the terminal nerve in a specimen of crown rump length 27 mm (7 weeks). Sagittal sections. Hematoxylin and eosin staining. Panels A and F display the lateral sites, while panels B and C show planes near the midsagittal plane. Thus, the terminal nerve (NT) is seen bilaterally (A, D). Panel F is 0.2 mm medial to Fig. 3F. Panel E is the higher magnification view of a square in panel D. Intervals between panels are 0.2 mm (A–B), 0.1 mm (B–C, C–D, D–F). The terminal nerve appears to be still closely related to the nerves to the vomeronasal organ (VNON) candidate (VNON? in panel B). In the immediately superior side of the cartilaginous nasal bone, the anterior ethmoidal nerve (AEN) crosses superficially the terminal nerve without communication (E, F). Panels A–D were prepared at the same magnification. Scale bars=1 mm (A), 0.1 mm (E, F).
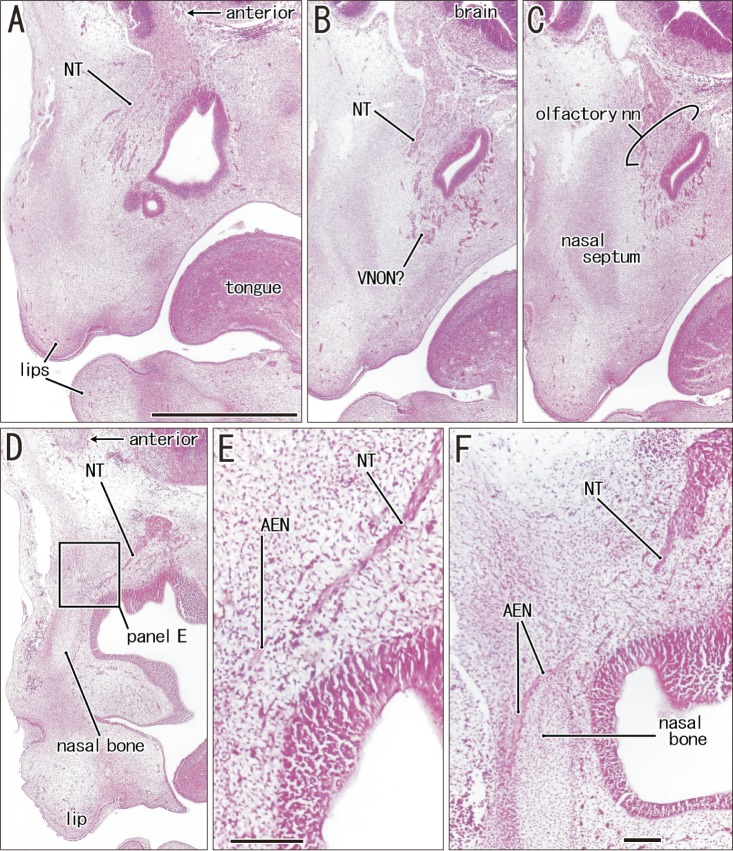
Fig. 5
Ganglion cells on the lateral surface of the crista galli of the ethmoid in a specimen of crown rump length 160 mm (18 weeks). Frontal sections. Panel A is 2.0 mm anterior to panel D. Panel B (or C) is a higher magnification view of a square in panel A (or B). Panel E is a higher magnification view of a square in panel D. Panel F is a section near panel E. Hematoxylin and eosin staining (A, F) or immunohistochemistry of neuron specific enolase (NSE) (B, C) and S100 protein (S100) (D, E). Ganglion cells on the crista galli are, 15–20 µm in diameter, positive for NSE (arrows in panels C), negative for S100 (arrows in panel E), and highly eosinophilic (arrows in panel F). Scale bars=1 mm (A, B, D), 0.1 mm (C, E, F). NS, nasal septum.
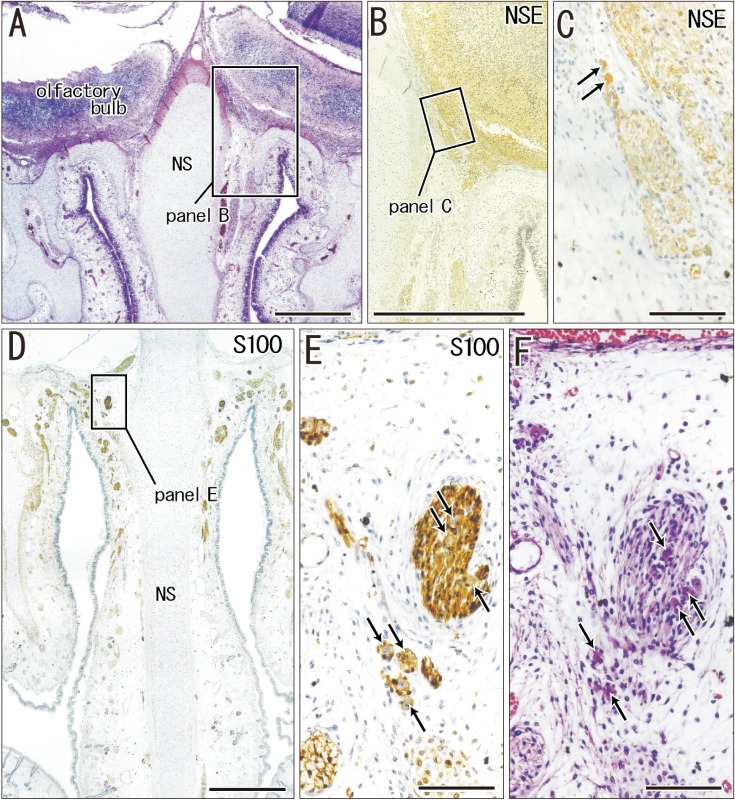
Table 1
Counting of ganglion cell bodies along the crista galli
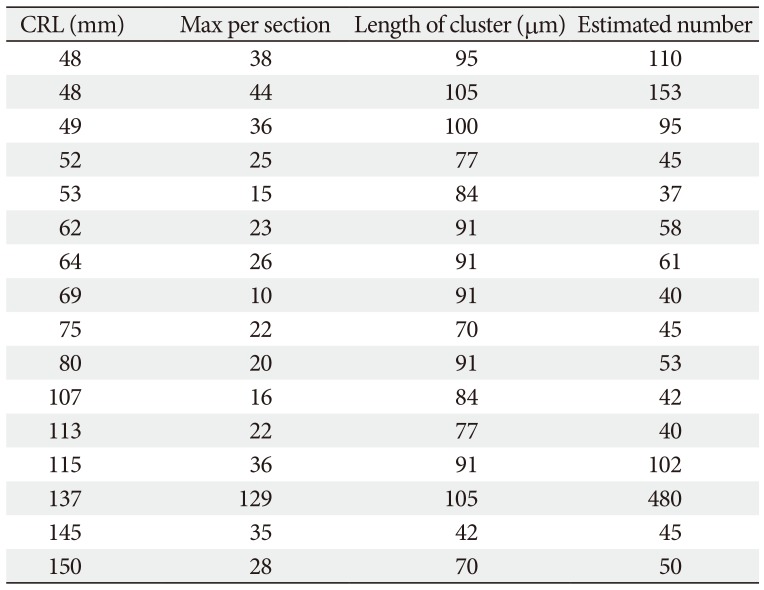
Max per section: maximum number of cell bodies per section (unilateral).
Length of cluster: an anteroposterior length of the ganglion cell cluster.
Estimated number (unilateral) is based on counting of cell bodies at 21-µm interval (1 of 3 sections of 7 µm in thickness).
There is no relationship between crown rump length (CRL) and max per section (r=0.350, P=0.184), CRL and length of cluster (r=−0.481, P=0.059), CRL and estimated number (r=0.260, P=0.330).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download