Introduction
Sexual dimorphism is the differences in traits between sexes of the same species, presumably due to evolution [
12]. This includes body “structures, shape and size due to inheritance in the genetic material of either of the sexual pattern” [
3]. Human skeletal structures and indeed teeth exhibit this inheritable genetic trait. Teeth are the most long-lasting structures in the body, withstanding the insults of bacterial degradation and fire, even more than bones [
456]. These make them ideal for fossil and evolutionary studies, and for human identification [
456].
There have been agitations by natives of the Niger-Delta area of Nigeria in which the Urhobo is a major ethnic group, as a result of environmental pollution occasioned by oil exploration. This had led to the spate of unidentified human remains as a result of kidnapping, killings and so forth by militia groups demanding development of host communities [
7].
Some studies have been conducted among different peoples of the world with a focus on dimorphic status of permanent maxillary molar crown or cusp, or combination of both [
8910111213141516].
However, there is paucity of studies on maxillary crown and cusps dimensions in the Nigerian population and indeed among Urhobo people, except for one conducted five years ago, with a focus on first maxillary crown dimensions [
16]. It has been stated, that differences in factors such as genetics, nutrition or physical development, that occur between races, ethnic groups or populations, impact on biological structures such as bone [
17], and teeth. Therefore, anthropometric values in one population may not accurately apply to another population in a different geographic location.
This study was undertaken to carry out anthropometric assessment of the dimensions of the crown and cusp of maxillary first molar (FM) and second molar (SM) for the purpose of sex determination among young Urhobo adults of Southsouth Nigeria. This study will be of relevance in forensic human identification, in the event that the teeth and indeed the molars are the only available structures to identify a dead or a missing person.
Go to :

Results
Results showed 55.9% (171) and 44.1% (135) of study subjects were males and females, respectively. The mean age of the subjects was 22.88±3.34 years.
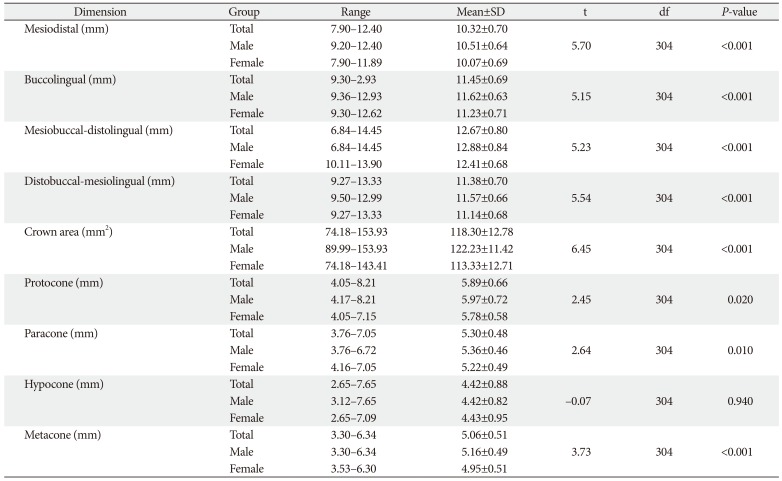
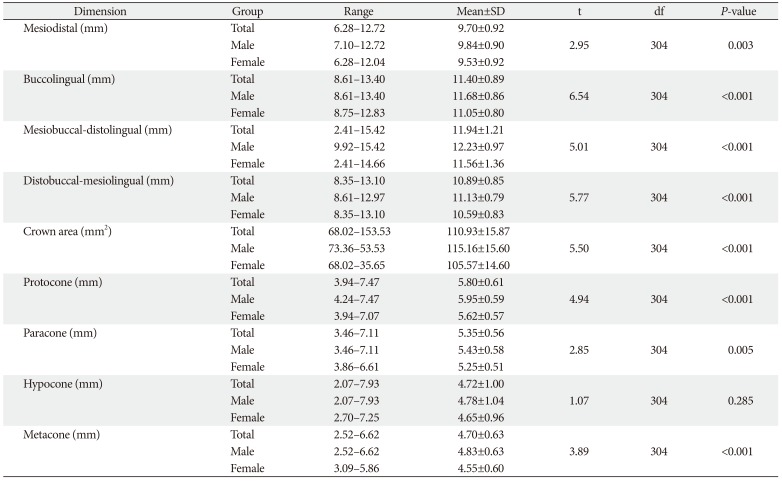
Tables 1 and
2 showed the various dimensions of maxillary FM and SM, respectively in both sexes and combined data. The BL was greater than MD in the maxillary first two molars. The mean DB-ML was also greater than MB-DL in the maxillary first two molars. In maxillary FM (
Fig. 1), mean cusp diameter was protocone>paracone>metacone>hypocone; while in maxillary SM (
Table 2), mean cusp diameter was protocone>paracone>metacone=hypocone. The mean crown dimensions were greater in the FM than in the SM, except paracone and hypocone that were greater in the maxillary SM.
Tables 1 and
2 also show comparison of the dimensions of the crown of maxillary FM and SM in both sexes. The mean values were significantly higher in males than females, except hypocone in which the differences were not significant.
Table 1
Comparison of crown dimensions of maxillary first molar between males and females
|
Dimension |
Group |
Range |
Mean±SD |
t |
df |
P-value |
|
Mesiodistal (mm) |
Total |
7.90–12.40 |
10.32±0.70 |
5.70 |
304 |
<0.001 |
|
Male |
9.20–12.40 |
10.51±0.64 |
|
Female |
7.90–11.89 |
10.07±0.69 |
|
Buccolingual (mm) |
Total |
9.30–2.93 |
11.45±0.69 |
5.15 |
304 |
<0.001 |
|
Male |
9.36–12.93 |
11.62±0.63 |
|
Female |
9.30–12.62 |
11.23±0.71 |
|
Mesiobuccal-distolingual (mm) |
Total |
6.84–14.45 |
12.67±0.80 |
5.23 |
304 |
<0.001 |
|
Male |
6.84–14.45 |
12.88±0.84 |
|
Female |
10.11–13.90 |
12.41±0.68 |
|
Distobuccal-mesiolingual (mm) |
Total |
9.27–13.33 |
11.38±0.70 |
5.54 |
304 |
<0.001 |
|
Male |
9.50–12.99 |
11.57±0.66 |
|
Female |
9.27–13.33 |
11.14±0.68 |
|
Crown area (mm2) |
Total |
74.18–153.93 |
118.30±12.78 |
6.45 |
304 |
<0.001 |
|
Male |
89.99–153.93 |
122.23±11.42 |
|
Female |
74.18–143.41 |
113.33±12.71 |
|
Protocone (mm) |
Total |
4.05–8.21 |
5.89±0.66 |
2.45 |
304 |
0.020 |
|
Male |
4.17–8.21 |
5.97±0.72 |
|
Female |
4.05–7.15 |
5.78±0.58 |
|
Paracone (mm) |
Total |
3.76–7.05 |
5.30±0.48 |
2.64 |
304 |
0.010 |
|
Male |
3.76–6.72 |
5.36±0.46 |
|
Female |
4.16–7.05 |
5.22±0.49 |
|
Hypocone (mm) |
Total |
2.65–7.65 |
4.42±0.88 |
−0.07 |
304 |
0.940 |
|
Male |
3.12–7.65 |
4.42±0.82 |
|
Female |
2.65–7.09 |
4.43±0.95 |
|
Metacone (mm) |
Total |
3.30–6.34 |
5.06±0.51 |
3.73 |
304 |
<0.001 |
|
Male |
3.30–6.34 |
5.16±0.49 |
|
Female |
3.53–6.30 |
4.95±0.51 |

Table 2
Comparison of crown dimensions of maxillary second molar between males and females
|
Dimension |
Group |
Range |
Mean±SD |
t |
df |
P-value |
|
Mesiodistal (mm) |
Total |
6.28–12.72 |
9.70±0.92 |
2.95 |
304 |
0.003 |
|
Male |
7.10–12.72 |
9.84±0.90 |
|
Female |
6.28–12.04 |
9.53±0.92 |
|
Buccolingual (mm) |
Total |
8.61–13.40 |
11.40±0.89 |
6.54 |
304 |
<0.001 |
|
Male |
8.61–13.40 |
11.68±0.86 |
|
Female |
8.75–12.83 |
11.05±0.80 |
|
Mesiobuccal-distolingual (mm) |
Total |
2.41–15.42 |
11.94±1.21 |
5.01 |
304 |
<0.001 |
|
Male |
9.92–15.42 |
12.23±0.97 |
|
Female |
2.41–14.66 |
11.56±1.36 |
|
Distobuccal-mesiolingual (mm) |
Total |
8.35–13.10 |
10.89±0.85 |
5.77 |
304 |
<0.001 |
|
Male |
8.61–12.97 |
11.13±0.79 |
|
Female |
8.35–13.10 |
10.59±0.83 |
|
Crown area (mm2) |
Total |
68.02–153.53 |
110.93±15.87 |
5.50 |
304 |
<0.001 |
|
Male |
73.36–53.53 |
115.16±15.60 |
|
Female |
68.02–35.65 |
105.57±14.60 |
|
Protocone (mm) |
Total |
3.94–7.47 |
5.80±0.61 |
4.94 |
304 |
<0.001 |
|
Male |
4.24–7.47 |
5.95±0.59 |
|
Female |
3.94–7.07 |
5.62±0.57 |
|
Paracone (mm) |
Total |
3.46–7.11 |
5.35±0.56 |
2.85 |
304 |
0.005 |
|
Male |
3.46–7.11 |
5.43±0.58 |
|
Female |
3.86–6.61 |
5.25±0.51 |
|
Hypocone (mm) |
Total |
2.07–7.93 |
4.72±1.00 |
1.07 |
304 |
0.285 |
|
Male |
2.07–7.93 |
4.78±1.04 |
|
Female |
2.70–7.25 |
4.65±0.96 |
|
Metacone (mm) |
Total |
2.52–6.62 |
4.70±0.63 |
3.89 |
304 |
<0.001 |
|
Male |
2.52–6.62 |
4.83±0.63 |
|
Female |
3.09–5.86 |
4.55±0.60 |

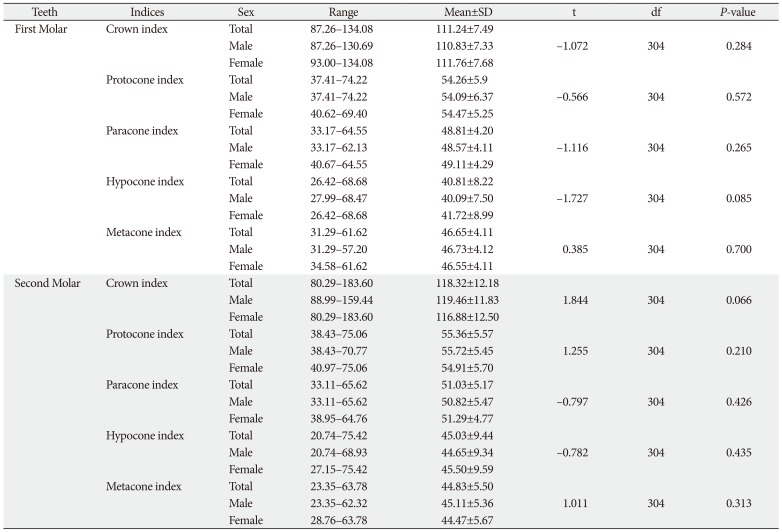
Table 3 showed the indices calculated in maxillary first two molars. The mean indices of the respective parameters were higher in FM compared to the SM, except for metacone index in which the reverse was the case. In all, no significant sex differences in crown and cusp indices were observed.
Table 3
Comparison of crown indices of 1st and second molars between the sexes
|
Teeth |
Indices |
Sex |
Range |
Mean±SD |
t |
df |
P-value |
|
First Molar |
Crown index |
Total |
87.26–134.08 |
111.24±7.49 |
−1.072 |
304 |
0.284 |
|
Male |
87.26–130.69 |
110.83±7.33 |
|
Female |
93.00–134.08 |
111.76±7.68 |
|
Protocone index |
Total |
37.41–74.22 |
54.26±5.9 |
−0.566 |
304 |
0.572 |
|
Male |
37.41–74.22 |
54.09±6.37 |
|
Female |
40.62–69.40 |
54.47±5.25 |
|
Paracone index |
Total |
33.17–64.55 |
48.81±4.20 |
−1.116 |
304 |
0.265 |
|
Male |
33.17–62.13 |
48.57±4.11 |
|
Female |
40.67–64.55 |
49.11±4.29 |
|
Hypocone index |
Total |
26.42–68.68 |
40.81±8.22 |
−1.727 |
304 |
0.085 |
|
Male |
27.99–68.47 |
40.09±7.50 |
|
Female |
26.42–68.68 |
41.72±8.99 |
|
Metacone index |
Total |
31.29–61.62 |
46.65±4.11 |
0.385 |
304 |
0.700 |
|
Male |
31.29–57.20 |
46.73±4.12 |
|
Female |
34.58–61.62 |
46.55±4.11 |
|
Second Molar |
Crown index |
Total |
80.29–183.60 |
118.32±12.18 |
1.844 |
304 |
0.066 |
|
Male |
88.99–159.44 |
119.46±11.83 |
|
Female |
80.29–183.60 |
116.88±12.50 |
|
Protocone index |
Total |
38.43–75.06 |
55.36±5.57 |
1.255 |
304 |
0.210 |
|
Male |
38.43–70.77 |
55.72±5.45 |
|
Female |
40.97–75.06 |
54.91±5.70 |
|
Paracone index |
Total |
33.11–65.62 |
51.03±5.17 |
−0.797 |
304 |
0.426 |
|
Male |
33.11–65.62 |
50.82±5.47 |
|
Female |
38.95–64.76 |
51.29±4.77 |
|
Hypocone index |
Total |
20.74–75.42 |
45.03±9.44 |
−0.782 |
304 |
0.435 |
|
Male |
20.74–68.93 |
44.65±9.34 |
|
Female |
27.15–75.42 |
45.50±9.59 |
|
Metacone index |
Total |
23.35–63.78 |
44.83±5.50 |
1.011 |
304 |
0.313 |
|
Male |
23.35–62.32 |
45.11±5.36 |
|
Female |
28.76–63.78 |
44.47±5.67 |

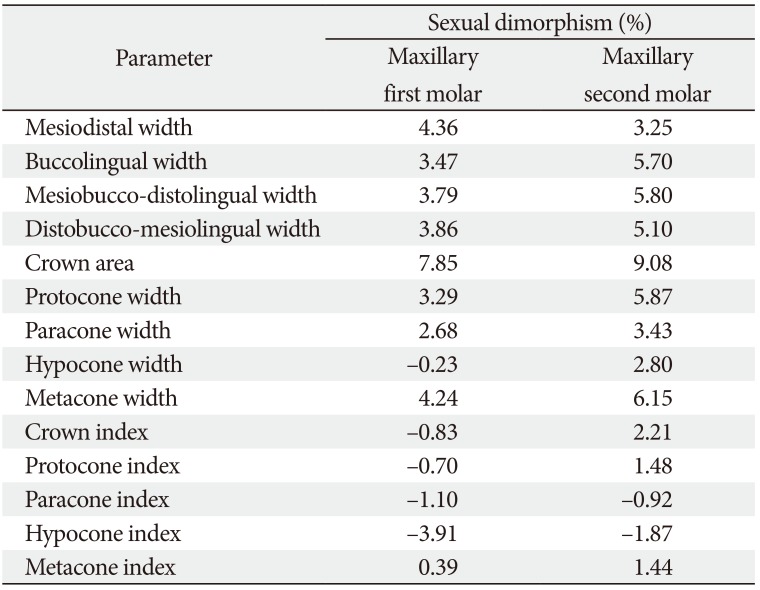
Table 4 showed the percentage sexual dimorphism of the various teeth dimensions measured. Considering sexual dimorphism in maxillary FM, MD>BL; MB-DL≈DB-ML; metac-one>protocone>paracone>hypocone. The result showed sexual dimorphism in maxillary SM dimensions as: BL>MD; MB-DL>DB-ML; metacone>protocone>paracone>hypocone. The level of sexual dimorphism was very low for all the indices in both molars, the highest being metacone index (0.39) and crown index (2.21) in FM and SM, respectively. When maxillary FM and SM were compared, sexual dimorphism in all parameters were higher for maxillary SM than maxillary FM, except in the case of MD in which FM was higher.
Table 4
Percentage sexual dimorphism of teeth parameters
|
Parameter |
Sexual dimorphism (%) |
|
Maxillary first molar |
Maxillary second molar |
|
Mesiodistal width |
4.36 |
3.25 |
|
Buccolingual width |
3.47 |
5.70 |
|
Mesiobucco-distolingual width |
3.79 |
5.80 |
|
Distobucco-mesiolingual width |
3.86 |
5.10 |
|
Crown area |
7.85 |
9.08 |
|
Protocone width |
3.29 |
5.87 |
|
Paracone width |
2.68 |
3.43 |
|
Hypocone width |
−0.23 |
2.80 |
|
Metacone width |
4.24 |
6.15 |
|
Crown index |
−0.83 |
2.21 |
|
Protocone index |
−0.70 |
1.48 |
|
Paracone index |
−1.10 |
−0.92 |
|
Hypocone index |
−3.91 |
−1.87 |
|
Metacone index |
0.39 |
1.44 |

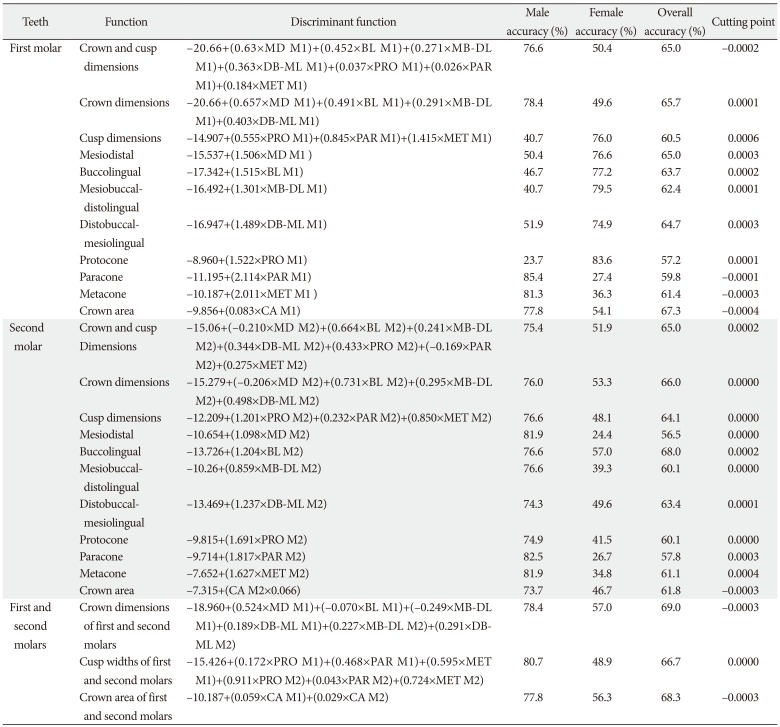
Discriminant function analyses were perfomed to predict whether an individual was either a male or a female. Predictor variables were mesiodistal, buccolingual, mesiodistalbuccolingual, distobuccal-mesiolingual dimensions; protocone, paracone, metacone and hypocone widths, crown areas of maxillary first two molars. The discriminant function is of the form:
D=k+c1x1+c2x2+…,.+cnxn; where D is the discriminant score, k is the Y-intercept, c is discriminant function coefficient, x is the discriminant variable raw score and n is the number of discriminant variable. Tests of equality of group means indicated significant differences for all discriminant variables except hypocones and all indices of both molars, which were excluded consequently.
The discriminant functions for sex determination from the first two molars and their combinations were shown in
Table 5. For FM, the highest overall accuracy of cross-validated assessment for crown dimensions was mesiodistal (65.0%) and distobuccal-mesiolingual (64.7%). The combination of crown dimensions only improved the outcome slightly (65.7%). Metacone gave the highest cross-validated overall assessment accuracy of all cusps, but when all cusp widths were combined, the outcome was poorer (60.5%). The combination of all crown dimensions and cusp widths could only give crossvalidated accuracy of 65.0%.
Table 5
Discriminant functions for sex determination from maxillary first two molars
|
Teeth |
Function |
Discriminant function |
Male accuracy (%) |
Female accuracy (%) |
Overall accuracy (%) |
Cutting point |
|
First molar |
Crown and cusp dimensions |
−20.66+(0.63×MD M1)+(0.452×BL M1)+(0.271×MB-DL M1)+(0.363×DB-ML M1)+(0.037×PRO M1)+(0.026×PAR M1)+(0.184×MET M1) |
76.6 |
50.4 |
65.0 |
−0.0002 |
|
Crown dimensions |
−20.66+(0.657×MD M1)+(0.491×BL M1)+(0.291×MB-DL M1)+(0.403×DB-ML M1) |
78.4 |
49.6 |
65.7 |
0.0001 |
|
Cusp dimensions |
−14.907+(0.555×PRO M1)+(0.845×PAR M1)+(1.415×MET M1) |
40.7 |
76.0 |
60.5 |
0.0006 |
|
Mesiodistal |
−15.537+(1.506×MD M1 ) |
50.4 |
76.6 |
65.0 |
0.0003 |
|
Buccolingual |
−17.342+(1.515×BL M1) |
46.7 |
77.2 |
63.7 |
0.0002 |
|
Mesiobuccaldistolingual |
−16.492+(1.301×MB-DL M1) |
40.7 |
79.5 |
62.4 |
0.0001 |
|
Distobuccalmesiolingual |
−16.947+(1.489×DB-ML M1) |
51.9 |
74.9 |
64.7 |
0.0003 |
|
Protocone |
−8.960+(1.522×PRO M1) |
23.7 |
83.6 |
57.2 |
0.0001 |
|
Paracone |
−11.195+(2.114×PAR M1) |
85.4 |
27.4 |
59.8 |
−0.0001 |
|
Metacone |
−10.187+(2.011×MET M1 ) |
81.3 |
36.3 |
61.4 |
−0.0003 |
|
Crown area |
−9.856+(0.083×CA M1) |
77.8 |
54.1 |
67.3 |
−0.0004 |
|
Second molar |
Crown and cusp Dimensions |
−15.06+(–0.210×MD M2)+(0.664×BL M2)+(0.241×MB-DL M2)+(0.344×DB-ML M2)+(0.433×PRO M2)+(–0.169×PAR M2)+(0.275×MET M2) |
75.4 |
51.9 |
65.0 |
0.0002 |
|
Crown dimensions |
−15.279+(–0.206×MD M2)+(0.731×BL M2)+(0.295×MB-DL M2)+(0.498×DB-ML M2) |
76.0 |
53.3 |
66.0 |
0.0000 |
|
Cusp dimensions |
−12.209+(1.201×PRO M2)+(0.232×PAR M2)+(0.850×MET M2) |
76.6 |
48.1 |
64.1 |
0.0000 |
|
Mesiodistal |
−10.654+(1.098×MD M2) |
81.9 |
24.4 |
56.5 |
0.0000 |
|
Buccolingual |
−13.726+(1.204×BL M2) |
76.6 |
57.0 |
68.0 |
0.0002 |
|
Mesiobuccaldistolingual |
−10.26+(0.859×MB-DL M2) |
76.6 |
39.3 |
60.1 |
0.0000 |
|
Distobuccalmesiolingual |
−13.469+(1.237×DB-ML M2) |
74.3 |
49.6 |
63.4 |
0.0001 |
|
Protocone |
−9.815+(1.691×PRO M2) |
74.9 |
41.5 |
60.1 |
0.0000 |
|
Paracone |
−9.714+(1.817×PAR M2) |
82.5 |
26.7 |
57.8 |
0.0003 |
|
Metacone |
−7.652+(1.627×MET M2) |
81.9 |
34.8 |
61.1 |
0.0004 |
|
Crown area |
−7.315+(CA M2×0.066) |
73.7 |
46.7 |
61.8 |
−0.0003 |
|
First and second molars |
Crown dimensions of first and second molars |
−18.960+(0.524×MD M1)+(–0.070×BL M1)+(–0.249×MB-DL M1)+(0.189×DB-ML M1)+(0.227×MB-DL M2)+(0.291×DBML M2) |
78.4 |
57.0 |
69.0 |
−0.0003 |
|
Cusp widths of first and second molars |
−15.426+(0.172×PRO M1)+(0.468×PAR M1)+(0.595×MET M1)+(0.911×PRO M2)+(0.043×PAR M2)+(0.724×MET M2) |
80.7 |
48.9 |
66.7 |
0.0000 |
|
Crown area of first and second molars |
−10.187+(0.059×CA M1)+(0.029×CA M2) |
77.8 |
56.3 |
68.3 |
−0.0003 |

Table 5 also showed that for the SM, buccolingual gave the highest overall accuracy of all crown dimensions (68.0%), but combination of all crown dimensions gave less accurate outcome (66.0%). Metacone gave the highest overall accuracy of all cusp widths (61.1%) and the combination of all cusp widths improved the overall outcome (64.1%).
The highest overall accuracy (69.0%) was achieved when all the crown dimensions of the first two molars were combined. Similarly when all the cusps (except hypocone) widths of the first two molars were combined, the outcome (66.7%) was higher than individual cusp assessments. On crown area, the overall accuracy of sex discrimination was higher when both molars were combined (68.3%) than when they were separated.
Go to :

Discussion
The present study presents data on buccolingual and mesiodistal widths and so forth both maxillary FM and SM molars concerning the Urhobo. The observation on the relative dimensions of the buccolingual and mesiodistal widths was in tandem with previous studies [
91213141920]. It was also consistent with Sharma et al. [
11] in the case of maxillary SM; but on the contrary, it was at variance with the latter in the case of maxillary FM. The reason for this may be because the MD is limited by teeth at both the mesial and distal contact points in contrast to the BL that does not have dental structures limiting it.
On diagonal widths, the result of the present study is similar to earlier reports [
81119] that MB-DL is greater than DB-ML in the first two maxillary molars.
On maximum diameter of the cusps, the first two maxillary molars exhibit same order with regards to their sizes, that is, from highest to lowest protocone followed by paracone, metacone, and hypocone. It is pertinent to state that in the present research, the earlier developed cusps were greater in size compared to the cusps that developed later. Prior studies reported variable results in different populations. Sharma et al. [
11] reported order of prominence in the first two maxillary molars from highest to lowest as hypocone, paracone, protocone and metacone, in a north Indian population. Among Indian Jat Sikhs, Agnihotri and Sikri [
13], reported hypocone>protocone>paracone>metacone, as the order of prominence of the cusp in the maxillary FM, just as was reported by Macaluso [
14] for the first two maxillary molars among black and white South Africans. Yadav et al. [
9] in a study of permanent maxillary FM cusp dimensions in Indians, reported the order of cusp size as hypocone>metacone>paracone>protocone. Among Libyan subjects, Sheikhi and Bugaighis [
19] reported the order of cusp sizes to be metacone> protocone>hypocone>paracone in the FM and protocone>metacone>hypocone>paracone in the SM. Differences in heredity and genes, race, ethnicity and environment may be the influencing factors in these studies.
The present study also showed that the MD, BL, MB-DL, and DB-ML were greater in maxillary FM compared with the SM. Clinical experience showed that the maxillary FM was larger than the SM, which was larger than the third and hence supports the above observation. Since the order of time of eruption of the molars is first, second and third molar, it is pertinent to assert that the crown of earlier erupted teeth is larger than the latter ones. Also, the above observation is consistent with earlier reports [
2122]. Nevertheless, the above assertion is also true for Zorba et al. [
12], but they also vary in the case of males in which the buccolingual crown dimension is greater in maxillary SM compared to the FM. This departure could be related to genetic and environmental factors as well as variation in error of measurements.
Results of the present study also indicate sexual dimorphism in all the dimensions of the crown and cusps measured, since male data were statistically significantly higher than female, except hypocone in the first two maxillary molars. This is consistent with previous studies by Yadav et al. [
9], Agnihori and Sikri [
13], Macaluso [
14], and Sheikhi and Bugaighis [
19], in a similar study among Libyan subjects, also reported statistically significant sexual dimorphism in all four crown dimensions in the first two maxillary molars, as well as protocone and metacone in the maxillary FM. However, no statistically significant sex difference in protocone and paracone of maxillary FM as well as all four cusps diameters in maxillary SM. Eboh [
16], in a study using the direct method of measurement among Urhobo people, reported statistical significant sexual dimorphism in mesiodistal and buccolingual widths on both sides, except the left maxillary mesiodistal width, of the FM. In a related study in India, Banerjee [
8] reported statistically significant differences between the sexes in mesiodistal and buccolingual widths of maxillary FM. Also in India, in Gujarati population, Bhavasar et al. [
20] reported that the right mesiodistal and left mesiodistal widths were statistically significantly higher in males compared to females, in contrast to the right and left bucculingual widths. The higher dentine in the crown of male teeth compared to female, as has been pointed out in the aforementioned studies could be the reason for the dimorphism.
On percentage sexual dimorphism, the MD followed by distobuccal-mesiolingual and mesiobuccal-distolingual widths showed the highest percentage of sexual dimorphism among all the crown dimensions of the maxillary FM, while protocone was highest among the cusps. Among maxillary SM crown dimensions, mesiobuccal-distolingual, buccolingual, followed by the distobuccal-mesiolingual widths, displayed the highest percentage sexual dimorphism, while metacone followed by protocone had the highest percentage sexual dimorphism of the cusps.
In Libyan subjects, Sheikhi and Bugaighis [
19] reported that buccolingual followed by the mesio-linguodistobuccal widths showed the highest percentage sexual dimorphism of all crown widths. They also observed that in descending order, percentage sexual dimorphism among the cusps in maxillary FM was paracone followed by protocone, hypocone, and metacone. They also reported that in maxillary SM, mesiobucco-distallingual followed by buccolingual widths displayed the highest level of sexual dimorphism among crown dimensions; while metacone followed by hypocone showed the highest percentage sexual dimorphism.
Eboh reported sexual dimorphism of 30% in buccolingual widths of both sides, as well as MD on the right, left maxillary FM being the least.
In a research by Zorba et al. [
12] the values of percentage sexual dimorphism in mesiodistal and buccolingual widths were higher than corresponding dimension in the present study. Also, sexual dimorphism was greater in left maxillary SM compared with FM. Sharma et al. [
11] reported percentage sexual dimorphism in descending order ML-DB, BL, MB-DL and MD, among crown dimensions of maxillary FM. They also observed that paracone and protocone were the highest, and metacone and hypocone, the lowest of the cusps in maxillary FM. In maxillary SM, MB-DL displayed the highest level of sexual dimorphism followed by BL; while hypocone is the highest among the cusps. Macaluso [
14] reported the order of sexual dimorphism of crown dimensions was BL>MD and MD>BL in maxillary FM and SM, respectively. In the case of cusp diameter, the order was protocone>hypocone>paracone>metacone and hypocone>protocone>paracone>metacone in maxillary FM and SM, respectively.
Agnihotri and Sikri [
13] reported the highest percentage sexual dimorphism in crown width to be BL followed by MD while among the cusps, the order from the highest was hypocone>metacone>protocone>paracone. The variable results reported in different studies from across the globe is an indication of variations in genetic, geographic or nutritional, and factors affecting anthropometry in human populations. It is also believed that the variable levels of sex dimorphism in the different studies may be as a result of same factors that affect human skeletal structures.
The level of sex discrimination of maxillary first two molar dimensions individually and in combination, is low to moderate, with overall classification accuracy of between 56.5% and 69.0%. These outcomes are comparable to those in prior studies. Agnihotri and Sikri [
13], Macaluso [
14] conducted a study on black South Africans and reported overall sex discriminatory accuracy in MD, BL combined MD and BL, protocone and paracone dimensions of maxillary FM higher than those of the present study, except metacone that are lower. In maxillary SM, except for BL that have equal overall accuracy with that of the present study, MD, combined MD and BL, protocone, paracone, and metacone dimensions had overall accuracy of sex discrimination higher than those of the present study. Similarly, the MD and BL for both FM and SM, and the combination of cusps in both FM and SM of black South Africans [
14] had higher overall sex discriminatory accuracy. Also, the overall sex discriminatory accuracy in a study among indigenous North Indians [
11] when all crown and cusp diameters were combined, were higher in the case of the FM but lower in the case of the SM when compared with the present study. In Libyan subjects [
19], the overall sex discriminatory accuracy when crown and cusp dimensions were combined were lower for both FM and SM when compared with those of present study. The variations observed between the present study and similar prior studies could be attributed to racial, ethnic, or population differences that affect anthropometric measurements; in addition, they could also be due to the different methods employed.
The present study reveals crown and cusp dimensions of the maxillary first two molars exhibit significant sexual dimorphism. It also established that in the maxillary FM, the crown dimension with the highest percentage sexual dimorphism is the mesiodistal and the least is buccolingual; while among cusp diameters, the highest is metacone and the least is protocone. In the maxillary SM, MB-DL and MD show the highest and least dimorphism in crown dimensions respectively, while protocone and hypocone displayed the highest and least sexual dimorphism respectively of the cusp diameters. In general, crown and cusp dimensions of the maxillary first two molars give good overall sex discriminatory accuracy; this implies that they can be employed in forensic anthropology for human sex determination.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download