Abstract
The ansa cervicalis is a neural loop in the neck formed by connecting the superior root from the cervical spinal nerves (C1–2) and the inferior root descending from C2–C3. It has various anatomical variations and can be an important acknowledgment in specific operations of the neck region. This is a review the anatomy, variations, pathology and clinical applications of the ansa cervicalis.
The ansa cervicalis (Fig. 1) innervates the infrahyoid muscles. “Ansa” is Latin for the handle of a cup [1]. It exists deep to the sternocleidomastoid and is a neural loop with two roots consisting of fibers from cervical ventral rami related to the cervical plexus. Generally, fibers arising from the ventral rami of cervical spinal nerves (C1–2) connect to the hypoglossal nerve within 3–4 cm. These then branch from the hypoglossal nerve and descend as the superior root of the ansa cervicalis. The inferior root of the ansa cervicalis is comprised of nerve fibers arising from ventral rami of C2–C3. These two branches join in the anterior wall of the carotid sheath and form a neural loop. The ansa cervicalis almost always travels anterior to the internal jugular vein [2]. This loop was previously called the “ansa hypoglossi” as it appeared to arise from the hypoglossal nerve and connect to the inferior root stemming from the cervical plexus [3]. This paper aims to review the anatomy, variations, and pathology of the ansa cervicalis and discuss its clinical applications.
The ansa cervicalis, which innervates the infrahyoid muscles, is a neural complex in the neck formed by the combination of the ventral rami of the first three or four cervical spinal nerves. It is comprised of both superior and inferior roots. The superior root of the ansa cervicalis, the ramus descendens hypoglossi, travels with the hypoglossal nerve and descends along the anterior wall of the carotid sheath. It contains only fibers from the first and second cervical spinal nerve (C1–2), not including the hypoglossal nerve [4]. After the superior root gives rise to a branch to the superior belly of the omohyoid, sternothyroid and sternohyoid muscles, it joins the inferior root arising from C2–3, forming the ansa cervicalis [3]. Blythe et al. [5] have described a rare case where the descendens hypoglossi (superior root) innervated the lower third of the sternocleidomastoid muscle. Chhetri and Berke [6] performed a literature review on the ansa cervicalis and found that the inferior root arises from the ventral rami of C2–3 in 74% of cases. In the majority of cases, the ansa cervicalis travels anterior to the internal jugular vein [2]. The inferior root gives rise to a branch that innervates the inferior belly of the omohyoid muscle which sometimes branches off of the fourth spinal cervical nerve (C4) [4] Another branch can be seen to descend into the thorax to join the cardiac or phrenic nerves [7].
The ansa cervicalis is considered an efferent nerve; however, there may be an afferent neural component to the infrahyoid muscles during phonation and deglutition [6]. Zapata and Torrealba [8] reported that reflex contraction of the cricothyroid muscle, mediated by afferent fibers, was induced by stimulation of the central end of the transected ramus descendens hypoglossi in cats. Additionally, previous studies have reported various connections between the ansa cervicalis, the cervical sympathetic trunk, with the descending branch of the ansa cervicalis linking a branch arising from C4 [91011121314].
Usually, the superior root of the ansa cervicalis travels along the anterior wall of the carotid sheath after leaving the hypoglossal nerve; however, the inferior root has a more varied composition and course, making the ansa cervicalis often asymmetric [15]. Caliot et al. [16] found the superior root was symmetric in almost all cases, however, the inferior root was asymmetric in 75% of the cases. They also described seven different forms of the ansa cervicalis in regard to the inferior roots: they are a simple classic form (27%), a very short single form (1.2%), a double classic form (40%), a double form with two separate roots (11%), a double short form (7.5%), a triple form (8.7%), and a quadruple form (1.2%) [16]. In addition, absence of the inferior root was reported 3% of the time [16]. Quadros et al. [17] have reported that ‘triple form’ of ansa cervicalis was unilaterally observed during the routine cadaveric dissection of the left neck. This case had three inferior roots originating from the accessory nerve, C1, C2, and C3 spinal nerves. A single inferior root formed between a branch from the accessory nerve and C1.
There is also a classification of the ansa cervicalis based on its relationship to the internal jugular vein [4]. There are three types: (1) the medial type where both roots of the ansa cervicalis are located deep to the internal jugular vein, (2) the lateral type in which the inferior root is anterior to the internal jugular vein [18], and (3) the mixed type where the ansa cervicalis has double loops; the upper branch of the inferior root runs posterior to the internal jugular vein while the lower root travels anterior to it. Moreover, Banneheka has classified different arrangements of the inferior root of ansa cervicalis into seven groups [19]. In all cases, the superior root of the ansa cervicalis was formed by C1–2. Group 1 forms a single loop between the superior root and the inferior root consisted of a single branch from either C2 or C3 (n=46, 22.1%). Group 2 was the most common variation where the inferior root had two branches joining the superior root independent of one another (n=86, 41.3%). Group 3 had the inferior root consisting of two branches, but their two branches joined the superior root at a single point (n=6, 2.9%). Group 4 showed two branches arising from C2, C3, or C4 joining to form one inferior root that formed a single loop with the superior root (n=58, 27.9%). Two branches arise from C2 or C3 in 53 cases and from C3 and C4 in five cases. Group 5 has the inferior root with three branches, which connect to the superior root independent of one another (n=6, 2.9%). Group 6 has the inferior root with three branches similar to group 5, but their three branches join the superior root at a single point (n=1, 0.5%) [20]. Group 7 has three branches arising from C2, C3, or C4 joining to form one inferior root which forms a single loop with the superior root (n=5, 2.4%).
Jelev classified the ansa cervicalis into five types [21]. It is further classified into three components for different neural communications: the C1–2 fibers with the hypoglossal nerve (hypoglossal component), the C1–2 fibers with the vagus nerve (vagal component), and separate branches off the C2–3 (cervical component). Type I does not form an ansa cervicalis. It has the hypoglossal component and cervical component without connection [22]. Type II is a typical ansa cervicalis (so-called hypoglosso-cervical ansa). The superior root branches from the hypoglossal component to form a loop with the inferior root from the cervical component. Type III has double loops and is composed of the hypoglosso-cervical ansa and vagocervical ansa (hypoglosso-vagocervical ansa) [232425]. Type IV has the hypoglossal component but forms a loop with the vagal and cervical components (vagocervical ansa) [2526272829]. Type V has the hypoglossal component and vagal component but does not form an ansa cervicalis [262830]. These authors have reported that, with the exception of type II, most variations of the ansa have a low frequency of less than 1%.
There are a few reports of unusual forms of the superior root of the ansa cervicalis. Verma et al. [31] have described an unusual case where the vagus and hypoglossal nerves were fused immediately after exiting the hypoglossal canal and jugular foramen, respectively. Branches from the vagus nerve innervated the sternohyoid, sternothyroid, and superior belly of the omohyoid muscles forming the superior root of the ansa cervicalis. Nayak et al. [32] have also reported a rare variant of the superior root branching off the vagus and hypoglossal nerves. Some variations lack the typical ansa cervical loop, forming the “vagocervical plexus” or “vagocervical complex” [3033]. It is a replacement of the ansa cervicalis formed by the vagus nerve and fibers from C1–2. The vagocervical plexus, which is a unilateral absence of the ansa cervicalis is a very rare finding [30]. In addition, a rare case was presented in which the ansa cervicalis was found to be totally absent on both sides, but was replaced by a vagocervical plexus [33]. The descending branch of this plexus entered the superior and inferior belly of the omohyoid, sternothyroid and sternohyoid muscles, distinguishing it from a pseudo ansa cervicalis, which does not give rise to muscular branches to the infrahyoid muscles. It could be considered a pseudo ansa cervicalis if the superior or inferior roots arise from the vagus nerve and the superior cervical sympathetic ganglion, respectively [34].
Banneheka [35] have observed communications between the ansa cervicalis and the vagus nerve using a surgical microscope. Two types of communications were observed: (1) false (pseudo) communications where two nerves were connected only by connective tissue, and (2) true communications, in which the two nerves were connected by nerve fibers. The majority of the ansa-vagal communications observed were false (pseudo) communications. The authors have suggested that the ansa-vagal communications result from the close physical relationship between the two nerves and may carry consideration in certain surgical procedures such as laryngeal reinnervation using the ansa cervicalis.
Schwannomas of the ansa cervicalis are rare occurrences [363738394041]. Schwannomas, also known as neuromas, neurilemmomas, or neurinomas, are benign nerve sheath tumor composed of Schwann cells [42]. Schwannomas occur most commonly in the head and neck regions arising from the vagus nerve or the sympathetic nervous system [43]. They are usually characterized by a painless mass in the neck and are difficult to diagnose because they are often mistaken for other lesions such as carotid body tumors, enlarged lymph nodes, branchial cysts, or thyroid lesions [4445]. Although de Diego Sastre et al. [37] reported a schwannoma of the ansa cervicalis, the preoperative diagnosis was a thyroid tumor [34].
The ansa cervicalis is an attractive and useful candidate for laryngeal reinnervation in cases of recurrent laryngeal nerve paralysis (RLNP) [46]. RLNP is one of the most serious complications in esophageal cancer surgery. Functional depression of deglutition and phonation induced by RLNP may lead to postoperative malnutrition and degradation of communication [47]. Moreover, RLNP may cause pneumonia by aspiration, affecting the long-term prognosis after esophageal cancer surgery [48]. Frazier [49] first reported laryngeal reinnervation using the ansa cervicalis for non-selective laryngeal reinnervation in 1924. It has been debated which branch of the ansa cervicalis should be used with many reporting that using the superior root improves surgical outcomes [49]. The collateral branches of the sternothyroid and omohyoid muscles have also been recommended for use in such operations [50]. Prades et al. [51] recommend the selection of the common nerve trunk to the sternothyroid and sternohyoid muscle as the prime choice. In 80% of the cases, it was in close proximity to the larynx and corresponded to the size of the recurrent laryngeal nerve. Recently, the application of the common trunk for laryngeal reinnervation was proposed as an easy, safe, and consistent method [52].
Iatrogenic damage to the ansa cervicalis may occur during surgical procedures such as thyroplasty [53], arytenoid adduction [54], Teflon injection [55], nerve-muscle pedicle implantation [56], surgery of the parotid gland, removal of the deep cervical lymph nodes [32], reanimation of facial paralysis using the hypoglossal nerve [57], and during infrahyoid myocutaneous flap reconstruction [58].
Figures and Tables
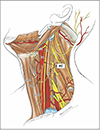
Fig. 1
Schematic drawing of anatomical structures of the ansa cervicalis in the left neck. The platysma, sternocleidomastoid, omohyoid muscle, all veins and the submandibular gland were already dissected. AC, ansa cervicalis; CCA, common carotid artery; IR, inferior root; OM, omohyoid muscle; PN, phrenic nerve; SHM, sternohyoid muscle; SR, superior root; STM, sternothyroid muscle; X, vagus nerve; XII, hypoglossal nerve.
References
1. Clemente CD. Neuroanatomy and neurophysiology. In : Landau SI, editor. International Dictionary of Medicine and Biology. New York: John Wiley;1986. p. 1917–1925.
2. Tubbs RS, Salter EG, Oakes WJ. Anatomic landmarks for nerves of the neck: a vade mecum for neurosurgeons. Neurosurgery. 2005; 56:2 Suppl. 256–260.

3. Chaurasia B. Human anatomy: regional and applied head and neck and brain. New Delhi: CBS Publishers;1980.
4. Olry R, Haines DE. Ansa hypoglossi or ansa cervicalis? That is the question. J Hist Neurosci. 2002; 11:302–304.

5. Blythe JN, Matharu J, Reuther WJ, Brennan PA. Innervation of the lower third of the sternocleidomastoid muscle by the ansa cervicalis through the C1 descendens hypoglossal branch: a previously unreported anatomical variant. Br J Oral Maxillofac Surg. 2015; 53:470–471.

6. Chhetri DK, Berke GS. Ansa cervicalis nerve: review of the topographic anatomy and morphology. Laryngoscope. 1997; 107:1366–1372.

7. Standring S. Gray's anatomy: the anatomical basis of clinical practice. 41st ed. New York: Elsevier;2016.
8. Zapata P, Torrealba G. Reflex effects evoked by stimulation of hypoglossal afferent fibers. Brain Res. 1988; 445:19–29.

9. Turner W. A phrenic nerve receiving a root of origin from the descendens hypoglossi. J Anat Physiol. 1893; 27(Pt 3):427.
10. Lippmann R. Abnormer Ursprung des Ramus descendens N. Anat Anz. 1910; 37:1–4.
11. Schaefer E, Symington J, Bryce T. Quain's elements of anatomy. 11th ed. London: Longmans, Green, and Co.;1915.
12. Rodrigues A. Le descendens cervicalis chez l'homme et chez le mammifères (quelques notes sur son évolution phylogénique). Assoc Anat Comptes Rendus. 1930; 25:267–282.
13. Winckler G. A propros des relations que relations que existent entre le plexus cervical et le nerf grand hypoglosse. Assoc Anat Comptes Rendus. 1955; 42:1415–1419.
14. Wischnewsky A. Die Aufbautypen des Ramus descendens nervi hypoglossi. Z Anat Entwicklungsgeschichte. 1930; 92:551–564.

15. Khaki AA, Shokouhi G, Shoja MM, Farahani RM, Zarrintan S, Khaki A, Montazam H, Tanoomand A, Tubbs RS. Ansa cervicalis as a variant of spinal accessory nerve plexus: a case report. Clin Anat. 2006; 19:540–543.

16. Caliot P, Dumont D, Bousquet V, Midy D. A note on the anastomoses between the hypoglossal nerve and the cervical plexus. Surg Radiol Anat. 1986; 8:75–79.

17. Quadros LS, Prasanna LC, D'Souza AS, Singh A, Kalthur SG. Unilateral anatomical variation of the ansa cervicalis. Australas Med J. 2015; 8:170–173.

18. Kikuchi T. A contribution to the morphology of the ansa cervicalis and the phrenic nerve. Kaibogaku Zasshi. 1970; 45:242–281.
19. Banneheka S, Tokita K, Kumaki K. Nerve fiber analysis of ansa cervicalis-vagus communications. Anat Sci Int. 2008; 83:145–151.

20. Machalek L, Charamza J, Kikalova K, Bezdekova M. A variant case of ansa cervicalis. Int J Anat Var. 2009; 2:150–152.
21. Jelev L. Some unusual types of formation of the ansa cervicalis in humans and proposal of a new morphological classification. Clin Anat. 2013; 26:961–965.

22. Venigopal SP, Mallula SB. Ansa cervicalis: without loop. Int J Anat Var. 2010; 3:153–155.
23. Rao TR, Shetty P, Rao SR. A rare case of formation of double ansa cervicalis. Neuroanatomy. 2007; 6:26–27.
24. Kumar N, Patil J, Mohandas R, Sirasanagandla , Nayak S, Guru A. Rare case of double looped ansa cervicalis associated with its deep position in the carotid triangle of the neck. Ann Med Health Sci Res. 2014; 4:Suppl 1. S29–S31.

25. Sangvichien S, Putsom O, Chuncharunee A. Anatomical variations of the ansa cervicalis in Thais. Siriraj Med J. 2003; 55:91–99.
26. Manjunath KY. Vagal origin of the ANSA cervicalis nerve: report of two cases. Indian J Otolaryngol Head Neck Surg. 2000; 52:257–258.
27. Vollala VR, Bhat SM, Nayak S, Raghunathan D, Samuel VP, Rodrigues V, Mathew JG. A rare origin of upper root of ansa cervicalis from vagus nerve: a case report. Neuroanatomy. 2005; 4:8–9.
28. D'Souza AS, Ray B. Study of the formation and distribution of the ansa cervicalis and its clinical significance. Eur J Anat. 2010; 14:143–148.
29. Ayyoubian M, Koruji M. A rare anatomical variant of ansa cervicalis: case report. Med J Islam Repub Iran. 2011; 24:238–240.
30. Rath G, Anand C. Vagocervical complex replacing an absent ansa cervicalis. Surg Radiol Anat. 1994; 16:441–443.

31. Verma R, Das S, Suri R. Unusual organization of the ansa cervicalis : a case report. Braz J Morphol Sci. 2005; 22:175–177.
32. Nayak SB, Shetty P, Reghunathan D, Aithal AP, Kumar N. Descendens vagohypoglossi: rare variant of the superior root of ansa cervicalis. Br J Oral Maxillofac Surg. 2017; 55:834–835.

33. Abu-Hijleh MF. Bilateral absence of ansa cervicalis replaced by vagocervical plexus: case report and literature review. Ann Anat. 2005; 187:121–125.

34. Indrasingh I, Vettivel S. A rare pseudo ansa cervicalis: a case report. J Anat Soc India. 2000; 49:178–179.
35. Banneheka S. Anatomy of the ansa cervicalis: nerve fiber analysis. Anat Sci Int. 2008; 83:61–67.

36. Hirabayashi S, Sakurai A, Fukuda O. Neurilemoma of the ansa cervicalis. Plast Reconstr Surg. 1987; 79:809–811.

37. de Diego Sastre JI, Melcón Díez E, Prim Espada MP. Neurilemmoma of the ansa cervicalis: a case report. Acta Otorrinolaringol Esp. 1996; 47:83–84.
39. Park JH, Ahn D, Hwang KH, Jeong JY. Schwannoma of ansa cervicalis in the submandibular space. Korean J Otorhinolaryngol-Head Neck Surg. 2014; 57:616–619.

40. Rath S, Sasmal PK, Saha K, Deep N, Mishra P, Mishra TS, Sharma R. Ancient Schwannoma of ansa cervicalis: a rare clinical entity and review of the literature. Case Rep Surg. 2015; 2015:578467.

41. Righini CA, Motto E, Faure C, Karkas A, Lefournier V, Reyt E. Schwannomas of the neck About 3 cases, and literature review. Rev Laryngol Otol Rhinol (Bord). 2007; 128:109–115.
42. Kang GC, Soo KC, Lim DT. Extracranial non-vestibular head and neck schwannomas: a ten-year experience. Ann Acad Med Singapore. 2007; 36:233–238.
43. Kim SH, Kim NH, Kim KR, Lee JH, Choi HS. Schwannoma in head and neck: preoperative imaging study and intracapsular enucleation for functional nerve preservation. Yonsei Med J. 2010; 51:938–942.

44. Kamal A, Abd El-Fattah AM, Tawfik A, Abdel Razek AA. Cervical sympathetic schwannoma with postoperative first bite syndrome. Eur Arch Otorhinolaryngol. 2007; 264:1109–1111.

45. Zhang H, Cai C, Wang S, Liu H, Ye Y, Chen X. Extracranial head and neck schwannomas: a clinical analysis of 33 patients. Laryngoscope. 2007; 117:278–281.

47. Baba M, Aikou T, Natsugoe S, Kusano C, Shimada M, Nakano S, Fukumoto T, Yoshinaka H. Quality of life following esophagectomy with three-field lymphadenectomy for carcinoma, focusing on its relationship to vocal cord palsy. Dis Esophagus. 1998; 11:28–34.

48. Loukas M, Thorsell A, Tubbs RS, Kapos T, Louis RG Jr, Vulis M, Hage R, Jordan R. The ansa cervicalis revisited. Folia Morphol (Warsz). 2007; 66:120–125.
49. Frazier CH. Anastomosis of the recurrent laryngeal nerve with the descendens noni: in cases of recurrent laryngeal paralysis. JAMA. 1924; 83:1637–1641.
51. Prades JM, Gavid M, Dubois MD, Dumollard JM, Timoshenko AT, Peoc'h M. Surgical anatomy of the ansa cervicalis nerve: which branch to use for laryngeal reinnervation in humans? Surg Radiol Anat. 2015; 37:139–145.

52. Chhetri DK, Blumin JH. Laryngeal reinnervation for unilateral vocal fold paralysis using ansa cervicalis nerve to recurrent laryngeal nerve anastomosis. Oper Tech Otolaryngol Head Neck Surg. 2012; 23:173–177.

53. Isshiki N, Okamura H, Ishikawa T. Thyroplasty type I (lateral compression) for dysphonia due to vocal cord paralysis or atrophy. Acta Otolaryngol. 1975; 80:465–473.

54. Isshiki N, Tanabe M, Sawada M. Arytenoid adduction for unilateral vocal cord paralysis. Arch Otolaryngol. 1978; 104:555–558.

55. Arnold GE. Vocal rehabilitation of paralytic dysphonia. VIII. Phoniatric methods of vocal compensation. Arch Otolaryngol. 1962; 76:76–83.

56. Tucker HM, Rusnov M. Laryngeal reinnervation for unilateral vocal cord paralysis: long-term results. Ann Otol Rhinol Laryngol. 1981; 90(5 Pt 1):457–459.

57. Yoleri L, Songür E, Yoleri O, Vural T, Cağdaş A. Reanimation of early facial paralysis with hypoglossal/facial end-to-side neurorrhaphy: a new approach. J Reconstr Microsurg. 2000; 16:347–355.

58. Deganello A, De Bree R, Dolivet G, Leemans CR. Infrahyoid myocutaneous flap reconstruction after wide local excision of a Merkel cell carcinoma. Acta Otorhinolaryngol Ital. 2005; 25:50–53.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download