Abstract
Anti-tumor necrosis factor (anti-TNF) is an effective biological agent for the treatment of moderate-to-severe active ulcerative colitis (UC) refractory to conventional therapy. On the other hand, anti-TNF therapy is strongly associated with a potential risk of tuberculosis (TB). Active TB is a critical complication that makes it difficult to treat patients who require anti-TNF for the treatment of UC refractory to conventional therapy. Based on the clinical guidelines, patients with inflammatory bowel disease (IBD) are strongly recommended to screen for latent TB before anti-TNF administration. Considering the possibility of active or reactivated TB related to anti-TNF therapy, all patients with IBD should be monitored closely for TB during anti-TNF therapy, irrespective of the screening results for latent TB. In particular, the risk of anti-TNF-related multidrug-resistant TB (MDR-TB) in patients with IBD has not been elucidated. This paper reports the first case of disseminated MDR-TB that developed in a UC patient receiving infliximab despite the negative evaluation for latent TB screening.
Infliximab is a chimeric IgG monoclonal antibody that blocks tumor necrosis factor-alpha (TNF-α). Anti-TNF, including infliximab, adalimumab, and golimumab, is effective in the treatment of patients with ulcerative colitis (UC) refractory to conventional treatment, such as corticosteroids and 5-aminosalicylic acid. On the other hand, anti-TNF therapy is strongly associated with the potential risk of tuberculosis (TB). Because TNF-α plays an important role in regulating the immune reactions and anti-TNF can reduce the number of chemokine secretions in response to mycobacteria infections, patients receiving infliximab therapy have an increased risk of a reactivation of latent TB. Therefore, patients with inflammatory bowel disease (IBD) should be screened for latent TB before starting anti-TNF therapy according to the clinical guidelines.1 Latent TB is diagnosed by a prior history of anti-TB medication and contact with active TB patients, chest X-ray, tuberculin skin test (TST), and/or interferon-gamma release assay (IGRA). All patients with IBD diagnosed with a latent TB should be treated with anti-TB medication before the initiation of anti-TNF therapy.2 On the other hand, the negative results for latent TB screening before anti-TNF therapy do not exclude the risk of active or reactivated TB in IBD patients on anti-TNF therapy.13 All patients receiving anti-TNF therapy for the treatment of IBD ought to be monitored closely for complicated TB, regardless of the negative evaluation for latent TB screening before starting anti-TNF therapy.
The lung is most common infected organ of TB, but extrapulmonary TB, such as lymphadenitis, urinary tract infection, osteoarthritis, meningitis, and intestinal TB, comprises 20% of TB cases worldwide.4 Disseminated TB is defined as the presence of TB that infects two or more noncontiguous organs. Multidrug-resistant TB (MDR-TB) is defined as the presence of infection caused by strains of Mycobacterium tuberculosis (M. tuberculosis) that are resistant to both isoniazid and rifampicin, which are the most potent agents for the treatment of TB. While the overall incidence of TB has declined, the incidence of MDR-TB increased from 3.8 to 5.9 cases per 100,000 person-years from 2000 to 2013.5 A literature review did not reveal any case of MDR-TB-related to anti-TNF therapy in patients with IBD. This paper reports the first case of disseminated MDR-TB that developed in a young female patient receiving infliximab for active UC refractory to conventional therapy, despite the negative evaluation for latent TB screening.
A 27-year-old woman, who had presented with abdominal pain, diarrhea, and hematochezia 9 months earlier, was diagnosed with UC at an outside hospital. She had never smoked, and her mean alcohol intake per week was 50 g. She had no underlying conditions and denied any family history of IBD and TB. She started intravenous hydrocortisone with mesalazine (Asacol®; Janssen Biotech, Horsham, PA, USA, 1.2 g per day) for induction therapy.
On the other hand, she did not enter remission after the induction therapy with steroids and mesalazine for UC. She was tested for latent TB screening considering infliximab administration for severe UC refractory to steroids. She denied a history of TB infection or anti-TB medications, as well as the possibility of exposure to any patient with known or suspected active TB. No evidence of latent or active TB was present based on QuantiFERON Gold test (QFT-G) and chest X-ray at the outside hospital. She had been treated with infliximab (Remicade®; Janssen Biotech, Horsham, PA, USA) 5 mg/kg intravenously twice with a 2-week interval and the dosage of mesalazine was increased to 3.2 g per day. Her symptoms improved after infliximab therapy, but she was referred to the Seoul National University Hospital because of her preference.
In terms of the UC Mayo subscore, her stool frequency was once or twice more than normal and rectal bleeding was observed one point at the initial evaluation. The clinical disease activity according to Truelove and Witt's criteria was moderate. The laboratory findings were as follows: white blood cell counts, 8,860/µL; hemoglobin, 8.0 g/dL; platelet counts, 569,000/µL; CRP, 0.60 mg/dL; erythrocyte sedimentation rate, 50 mm/hr; and fecal calprotectin, 215 µg/g. Her liver function tests and estimated glomerular filtration rate were normal. The serological markers of hepatitis B and C, anti-neutrophil cytoplasmic antibody titer, and anti-saccharomyces cerevisiae antibody were all negative. The results of IGRA (QFT-G) and chest X-ray were also negative. On the follow-up colonoscopy examination, mild focal erythema, and edema with inflammatory pseudopolyps were detected from the cecum to the ascending colon with multifocal fibrotic scars from the sigmoid colon to the rectum, suggesting mucosal healing with a Mayo endoscopic subscore of one point (Fig. 1). Infliximab with mesalazine therapy was continued based on the good therapeutic response.
After 6 months of starting infliximab therapy, she developed progressive flu-like symptoms, including high fever, chilling sense, and generalized myalgia. She also had a dry cough, abdominal distension, and dyspepsia. On the other hand, she denied bloody diarrhea and the average bowel movement was once a day. Upon hospitalization, her vital signs were as follows: a body temperature of 37.5℃, blood pressure of 140/94 mmHg, heart rate of 107/min, and respiratory rate of 18/min. No abnormal findings were observed on the physical examination except for abdominal distension with positive shifting dullness and mild tenderness around the lower abdomen. The laboratory results were as follows: white blood cell counts, 5,040/µL; hemoglobin, 9.7 g/dL; platelet counts, 402,000/µL; CRP, 11.80 mg/dL; and erythrocyte sedimentation rate, 77 mm/hr. CT of the chest, abdomen, and pelvis revealed enlarged lymph nodes in the right paratracheal and supraclavicular area, and a large volume of ascites with diffuse peritoneal thickening that was compatible with TB peritonitis accompanied by lymphadenitis (Fig. 2). The examination for ascites revealed a low serum ascites albumin gradient with a lympho-dominant profile. The ascitic adenosine deaminase (ADA) level was 154 IU/L (normal range, 5.3–17.8 IU/L). The results of the ascitic acid-fast bacilli (AFB) smear and culture, and TB and non-tuberculous mycobacteria PCR were all negative. The re-test IGRA was also negative. Bronchoscopy and endobronchial ultrasound-guided fine needle biopsy were performed from the right paratracheal lymph node to confirm the presence of TB lymphadenitis. No abnormal findings suggesting endobronchial TB were noted on the bronchoscopic evaluation. The histology examination of the lymph node biopsy specimens revealed chronic granuloma with multinucleated giant cells that suggested the possibility of TB lymphadenitis (Fig. 3). The AFB culture of the lymph node was positive for M. tuberculosis and the Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) assay was also positive for M. tuberculosis resistant to rifampin. In addition, the AFB culture of the sputum samples was also positive for M. tuberculosis on the broth medium. Infliximab therapy was discontinued due to the development of complicated TB.
Because M. tuberculosis resistant to rifampin is more likely to have a concomitant resistance to isoniazid,6 she received a combination of anti-TB drugs with intramuscular kanamycin 1 g every 24 hours, oral levofloxacin 750 mg per day, prothionamide 1,500 mg per day, cycloserine 250 mg three times per day, and pyridoxine 50 mg three times per day for suspicious disseminated MDR-TB based on the consultation with a TB specialist. Her symptoms were relieved gradually, and she was discharged after 13 days of hospitalization. After 4 weeks of the anti-TB drugs, however, she was re-hospitalized for cramping abdominal pain and vomiting developed. Abdominal radiography and ultrasound showed gaseous distention of the small bowel, suggesting paralytic ileus associated with MDR-TB peritonitis. Intravenous agents, including linezolid 600 mg every 24 hours, meropenem 1 g every 8 hours, and oral clavulanic acid 625 mg every 8 hours were added considering the insufficient efficacy of the previous regimens for MDR-TB peritonitis. The patient was discharged with an improvement of her symptoms on hospital day 26. All symptoms related to MDR-TB subsided 9 weeks after starting anti-TB medication.
Approximately 3 months after the initiation of anti-TB therapy, the results of the drug sensitivity test (DST) for M. tuberculosis from the sputum specimen on the broth medium were as follows: resistance to all first-line agents for TB, including isoniazid, rifampicin, ethambutol, and pyrazinamide, as well as streptomycin and quinolone. Based on the results of DST, the regimens were switched to intramuscular kanamycin 1 g five times per week, oral prothionamide 250 mg three times per day, cycloserine 250 mg three times per day, linezolid 300 mg once per day, and delamanid 100 mg twice per day. Kanamycin was discontinued due to an adverse effect of paresthesia 9 months after the initiation of administration.
The follow-up sputum AFB smear and culture results were all negative 3 weeks ago after starting the anti-TB medications. She had quiescent TB during 12 months of anti-TB medication. She also maintained the clinical remission of UC using mesalazine (Pentasa® SR; Ferring AS, Vanlese, Denmark, 2 g twice per day) with probiotics (Saccharomyces boulardii, Bioflor®; Kuhnil Pharmaco. Co., Seoul, Korea, 250 mg 3 times per day) after discontinuing the infliximab therapy. Fig. 4 summarizes her clinical course.
The 27-year-old female patient with steroid-refractory UC experienced disseminated MDR-TB involving the lung, peritoneal cavity, and multiple lymph nodes 27 weeks after the initiation of infliximab, despite the negative results for latent TB screening. She improved after being administered multiple therapeutic regimens for MDR-TB with the discontinuation of infliximab. Moreover, she did not experience clinical relapse within 12 months after the discontinuation of infliximab. A literature review revealed IBD patients on anti-TNF therapy to have a higher risk of extrapulmonary or disseminated TB than the general population.2 In a Groupe d'Etude Thérapeutique des Affections Inflammatoires du tube Digestif (GETAID) cohort study, 91% of cases of anti-TNF-associated TB involved at least one extrapulmonary site.3 In a recent Korean multicenter observational study, 25 cases (2.9%) of TB developed among 873 patients with IBD receiving anti-TNF agents.7 Among them, three cases (12%) were pulmonary MDR-TB, but there was no case of disseminated TB resistant to both isoniazid and rifampicin. In the present case, however, primary MDR-TB developed in both lung and multiple extrapulmonary organs of a patient with UC receiving infliximab. Similar to this case, some patients with IBD can experience the development of anti-TNF-related TB, even though their results for latent TB screening were negative before starting anti-TNF. The mean interval between the first exposure of infliximab and the diagnosis of TB ranges from 8 to 19 weeks.89 Approximately 50% of individuals infected with TB develop active TB within 1 to 2 years after the infection, but the remaining half can develop at any time in their lifetime.
One hypothesis of the complicated TB related to infliximab despite the negative evaluation for latent TB is that the screening results using chest X-ray and IGRA test were a false negative. IGRA includes QFT-G, QuantiFERON®-TB Gold In Tube (Cellestis limited, Carnegie Victoria, Australia), and T-SPOT®.TB (Oxford Immunotec Ltd., Abingdon, UK). The sensitivity of QFT-G for latent TB was 75% (95% CI, 66–82%).10 Immunemediated diseases using immunosuppressive agents are closely related to the decreased sensitivity of QFT-G for latent TB. In lymphocyte-deficient patients, the sensitivity of QFT-G for latent TB was only 39% (95% CI, 21–57%).11 IGRA demonstrates immune sensitization by detecting a cell-mediated immune response by stimulation with mycobacterial antigens. Recent steroid prescription can induce indeterminate results of IGRA. Therefore, IBD patients receiving immunosuppressive agents can produce inaccurate results of IGRA for latent TB. The screening results for latent TB using IGRA should be interpreted carefully in IBD patients, particularly those taking immunosuppressive agents, such as steroids.
The other hypothesis is that the patient without latent TB comes in contact with patients with active TB during anti-TNF therapy. Patients with infectious TB can spread infectious droplets to others even with light contact, such as talking for 5 minutes or coughing once. Approximately 3.3% of household contacts of MDR-TB and 4.8% of household contact of drug-susceptible TB resulted in the development of active TB.12 South Korea, in which the incidence of active TB was 77 per 100,000 persons in 2016, has a high burden of TB despite the national and full-scale efforts to eradicate TB infections. The incidence of latent TB among healthcare workers was at least 3% per year,13 which makes it difficult to control TB infections.
TB peritonitis is an unusual manifestation of TB that occurs through hematogenous spread from the lung and the reactivation of a latent TB in the peritoneum. The diagnosis of TB peritonitis remains a challenge for clinicians because of the difficulty in diagnosing this condition. The gold standard for the diagnosis of TB peritonitis is an ascitic fluid or peritoneal biopsy culture positive for M. tuberculosis. On the other hand, it might take more than 4 weeks with a modest sensitivity of 66–83% in as much as 1 L of ascitic fluid.14 The sensitivity of an AFB culture of laparoscopic biopsy for TB peritonitis is 93%, but the biopsy is invasive and expensive. Therefore, alternative non-invasive diagnostic tools, including AFB staining, PCR and ADA level in the ascitic fluid, and IGRA, are used for the early detection of TB peritonitis. Nevertheless, the diagnostic sensitivity of an ascitic fluid AFB smear is only 2.9% and PCR for M. tuberculosis reveals a low sensitivity of 48% in smear-negative ascites.15 An ascitic fluid ADA activity of more than 30 IU/L is accepted as the cut-off level expected to provide the highest diagnostic yields for TB peritonitis, with a sensitivity and specificity of more than 90%.14 On the other hand, the high ADA in the ascites does not always mean TB peritonitis. Instead of the microbiological diagnosis, radiology images, such as abdominal ultrasound and CT, may be helpful in diagnosing TB peritonitis as well as in evaluating the involvement of other extrapulmonary organs.
TB lymphadenitis is the most common manifestation of extrapulmonary TB. A definite diagnosis of TB lymphadenitis can be made by the demonstration of M. tuberculosis through an AFB culture or PCR of the affected lymph node. An excisional biopsy is the most sensitive but most invasive diagnostic test. On the other hand, needle aspiration and biopsy are safer and more practical than an excisional biopsy, especially when a surgical approach is unavailable. In this case, bronchoscopic lymph node biopsy was performed using an Xpert MTB/RIF assay to define the presence of TB lymphadenitis in the mediastinum. The Xpert assay is a validated diagnostic tool for extrapulmonary TB regardless of the microbiological confirmation.16 IGRA is also a valuable diagnostic tool for both extrapulmonary and pulmonary TB. The sensitivity for the diagnosis of extrapulmonary TB was 72% (95% CI, 65–79%) for QFT-G or QuantiFERON Gold in-tube test.17 On the other hand, further investigations will be needed to evaluate the utility of IGRA depending on the sites of TB involvement.
In this case, M. tuberculosis was detected from the sputum AFB culture despite normal chest X-ray findings. All suspected extrapulmonary TB patients are required to evaluate the possibility of concomitant lung involvement because pulmonary TB is also present in approximately 10–50% of extrapulmonary TB patients.18 Some patients with extrapulmonary TB can have a positive sputum culture for M. tuberculosis despite the normal chest X-ray. In a retrospective study, there was no significant difference in the positivity of a sputum culture for M. tuberculosis from extrapulmonary TB patients between abnormal and normal chest X-ray findings.19
Rifampin resistance was confirmed by an Xpert assay in this case. In terms of the treatment of rifampicin-resistant TB, clinicians should consider the administration of second- line anti-TB medications for MDR-TB because approximately 90% of rifampin-resistant specimens also showed isoniazid resistance. The regimen for the MDR-TB treatment should be individualized based on the patients' DST results, and at least four susceptible drugs should be included. The duration of MDR-TB treatment can also be tailored. A combination of oral and injectable agents for 9 to 12 months is recommended in patients with MDR-TB.20
In a retrospective study from India, which has high endemicity of TB, UC patients with TB on anti-TNF therapy showed good responses to anti-TB medication and remained in the remission state for the next 24 months after discontinuing the anti-TNF therapy,8 which is consistent with the present case. Therefore, the clinical outcomes of UC in patients who develop complicated TB-related to anti-TNF might be promising, even if the anti-TNF is discontinued. Patients with complicated TB should not take anti-TNF before the complicated TB is cured. On the other hand, resuming anti-TNF after the completion of anti-TNF medications can be considered because approximately 50% restarted anti-TNF therapy after the completion of anti-TB drugs. On the other hand, none experienced a relapse of TB during the follow-up periods in the GETAID cohort study of participants diagnosed with TB after the first anti-TNF therapy.3
One limitation of this case was that TST was not performed for the latent TB screening. On the other hand, the specificity of TST for the detection of latent TB is attenuated by Bacillus Calmette-Guérin (BCG) vaccination, which is strongly recommended for all infants according to the national immunization program of South Korea. Therefore, the utility of TST for latent TB screening appears to be low.
This paper reports the first case of a patient with UC who was diagnosed with primary disseminated MDR-TB related to infliximab therapy despite the negative evaluation for latent TB before the initiation of infliximab. IBD patients receiving anti-TNF therapy should be monitored carefully in a region with a high prevalence of TB, irrespective of the screening result for latent TB before anti-TNF therapy. The possibility of primary MDR-TB should be considered during the diagnosis of complicated TB related to anti-TNF therapy based on the increasing trend in the incidence of MDR-TB worldwide.
Figures and Tables
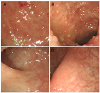 | Fig. 1Colonoscopy exams showed (A, B) mild erythema, edema, and exudate with inflammatory pseudopolyps were noted from the cecum to the ascending colon, and (C, D) multiple whitish scars were found from the sigmoid colon to the rectum. |
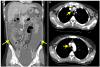 | Fig. 2Computed tomography of the chest, abdomen and pelvis showed (A) a large volume of ascites with diffuse peritoneal thickening (arrows), which are consistent with tuberculous peritonitis, and (B) enlarged lymph nodes in the right upper and lower paratracheal area (arrows). |
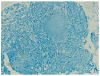 | Fig. 3Acid-fast bacilli (AFB) stain of a paratracheal lymph node biopsy specimen showed granulomatous inflammation with multinucleated giant cells. AFB were not detected in the specimen (AFB, ×400). |
 | Fig. 4Clinical course. She experienced disseminated multidrug-resistant tuberculosis involved in the lung, peritoneal cavity, and multiple lymph nodes 27 weeks after the initiation of infliximab for the treatment of ulcerative colitis, despite the negative results for latent tuberculosis screening. UC, ulcerative colitis; MDR-TB, multidrug-resistant tuberculosis; W, week; Km, Kanamycin; Lfx, levofloxacin; Pto, prothionamide; Cs, cycloserine; Lzd, linezolid. |
References
1. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res. 2018; 16:4–16.

2. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management. Intest Res. 2018; 16:17–25.

3. Abitbol Y, Laharie D, Cosnes J, et al. Negative screening does not rule out the risk of tuberculosis in patients with inflammatory bowel disease undergoing anti-TNF treatment: a descriptive study on the GETAID cohort. J Crohns Colitis. 2016; 10:1179–1185.

4. Ribeiro S, Trabulo D, Cardoso C, Oliveira A, Cremers I. Disseminated tuberculosis in an immunocompetent patient: the answer is in the liver. GE Port J Gastroenterol. 2015; 23:208–213.

5. Kendall EA, Azman AS, Cobelens FG, Dowdy DW. MDR-TB treatment as prevention: the projected population-level impact of expanded treatment for multidrug-resistant tuberculosis. PLoS One. 2017; 12:e0172748.

6. Kurbatova EV, Cavanaugh JS, Shah NS, et al. Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other anti-tuberculosis drugs. Int J Tuberc Lung Dis. 2012; 16:355–357.
7. Byun JM, Lee CK, Rhee SY, et al. Risks for opportunistic tuberculosis infection in a cohort of 873 patients with inflammatory bowel disease receiving a tumor necrosis factor-α inhibitor. Scand J Gastroenterol. 2015; 50:312–320.

8. Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: experience from India-a country with high prevalence of tuberculosis. J Gastroenterol Hepatol. 2017; 32:1191–1194.

9. Agarwal A, Kedia S, Jain S, et al. High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India. Intest Res. 2018; 16:588–598.

10. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008; 149:177–184.

11. Komiya K, Ariga H, Nagai H, et al. Impact of peripheral lymphocyte count on the sensitivity of 2 IFN-gamma release assays, QFT-G and ELISPOT, in patients with pulmonary tuberculosis. Intern Med. 2010; 49:1849–1855.

12. Grandjean L, Gilman RH, Martin L, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med. 2015; 12:e1001843.

13. Yeon JH, Seong H, Hur H, et al. Prevalence and risk factors of latent tuberculosis among Korean healthcare workers using whole-blood interferon-γ release assay. Sci Rep. 2018; 8:10113.

14. Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005; 22:685–700.
15. Rapid diagnostic tests for tuberculosis: what is the appropriate use? American Thoracic Society Workshop. Am J Respir Crit Care Med. 1997; 155:1804–1814.
16. Lombardi G, Di Gregori V, Girometti N, Tadolini M, Bisognin F, Dal Monte P. Diagnosis of smear-negative tuberculosis is greatly improved by Xpert MTB/RIF. PLoS One. 2017; 12:e0176186.

17. Fan L, Chen Z, Hao XH, Hu ZY, Xiao HP. Interferon-gamma release assays for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. FEMS Immunol Med Microbiol. 2012; 65:456–466.

18. Mota PC, Sá D, Mota M, Duarte R. Extrapulmonary tuberculosis: importance of differential diagnosis. BMJ Case Rep. 2011; 2011:bcr0620114363.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download