Abstract
Eosinophilic gastrointestinal disorder (EGID) is an uncommon disease that is accompanied by intestinal eosinophil infiltration without a secondary cause of eosinophilia. Eosinophilic enteritis is a secondary portion of EGID that can present a range of gastrointestinal symptoms according to the affected depth of the intestinal layer. The subserosal type of eosinophilic enteritis presenting as ascites is relatively rarer than the mucosal type. In general, eosinophilic enteritis occurs in patients with food allergies, but its mechanism is unclear. The authors experienced a 29-year-old female patient with a large amount of ascites with diarrhea and abdominal pain. The patient was diagnosed with an influenza A infection one week earlier. Peripheral eosinophilia (absolute eosinophil count: 6,351 cells/mm3) and eosinophilic ascites (97% of white blood cells in the ascites are eosinophil) were present. Abdominal CT revealed a large amount of ascites and edematous changes in the ileum and ascending colon wall. A diagnosis of eosinophilic enteritis was confirmed as eosinophilic ascites by paracentesis, with eosinophil infiltration of the bowel wall by an endoscopic biopsy. The patient's symptoms improved rapidly after using steroids. To the best of the author's knowledge, this is the first report of eosinophilic enteritis with massive ascites after an influenza A virus infection in a Korean adult.
Eosinophilic gastrointestinal disorder (EGID) is an uncommon disease accompanied by intestinal eosinophil infiltration without a secondary cause of eosinophilia.12 The definition of EGID is as follows: 1) abnormal gastrointestinal symptoms, such as abdominal pain and diarrhea; 2) histopathological evidence of eosinophil infiltration at only the gastrointestinal tract; and 3) no evidence of parasite infection or malignancy.23 Eosinophilic enteritis is a secondary portion of EGID that can present a range of gastrointestinal symptoms according to the affected depth of the intestinal layer.245 The subserosal type of eosinophilic enteritis presenting as ascites is relatively rarer than the mucosal type. In general, eosinophilic enteritis occurs in patients with food allergies, but its mechanism is unclear.
A previous study reported eosinophilic enteritis caused by an influenza A infection in a child without allergies.6 This paper reports a case of eosinophilic enteritis with a large amount of ascites after an influenza A virus infection in an adult with allergic rhinitis.
A 29-year-old female patient visited the outpatient clinic with ascites. One week earlier, she was admitted to a local hospital because of myalgia, fever, abdominal pain, and diarrhea. She was diagnosed with an influenza A virus infection by RT-PCR from a throat swab specimen. Peramivir (Peramiflu®; Green Cross, Yongin, Korea) was administered. An abdominal ultrasound was performed to evaluate the sustained abdominal pain. A large amount of ascites were found. The patient had no history of alcohol consumption or smoking. In her past medical history, she had been diagnosed with allergic rhinitis against cats seven years ago, but she was untreated and kept in constant contact with her domestic cat. She did not appear very unwell when she visited the outpatient clinic and she was alert. The patient had an initial blood pressure of 120/90 mmHg, pulse rate of 82 beats/min, body temperature of 36. 5℃, and respiratory rate of 20 breaths/min. Her abdomen was distended and bowel sound was increased mildly. Non-specific findings were noted, except for a shift in abdominal dullness on the abdominal physical examination.
The laboratory test results were as follows: white blood cell count of 14,400 cells/mm3 with 44.8% eosinophils (absolute eosinophil count: 6,351 cells/mm3) and 32.7% neutrophils, hemoglobin level of 14.0 g/dL, and platelet count of 368×103 cells/mm3. No abnormality of electrolytes was noted. She had protein, albumin, BUN, and creatinine levels of 6.6 g/dL, 4.0 g/dL, 6.2 mg/dL, and 0.69 mg/dL, respectively, as well as an erythrocyte sedimentation rate and CRP level of 9 mm/hr and 0.48 mg/dL, respectively. The antigen of the hepatitis B surface and antibody against hepatitis C were both negative. Serum CA 125 and AFP level were 368.9 µg/mL (reference range: 0–35 µg/mL) and 2.2 ng/mL (reference range: 0–8.1 ng/mL), respectively. No proteinuria or albuminuria was found at urine analysis. No specific findings in the peripheral blood smear test performed to exclude hematological malignancy.
A parasite test and rheumatoid factor test were performed to identify the cause of the eosinophilia. The parasite specific IgG test and stool test for parasites were negative. The toxoplasma test was also negative. The anti-nuclear antibody and result of other autoimmune disease laboratory test were all negative. A total IgE and multiple allergen simultaneous test were performed to determine the presence and cause of the allergy. The total IgE was increased to 375.7 IU/mL (reference range: 0–35 IU/mL). The multiple allergen simultaneous test for cats showed a 4+ finding for cats.
A bilateral diaphragmatic elevation was observed on the chest X-ray, and diffuse haziness was observed on the abdominal X-ray due to the large amount of ascites. Abdominal CT also revealed massive ascites, edematous changes to the ileum and ascending colon wall (Fig. 1). No cirrhotic findings were noted on abdominal ultrasonography. A large amount of ascites was observed by ultrasonography. Diagnostic paracentesis was performed. The ascitic fluid color was orange in color with increased turbidity. The laboratory test results of the ascitic fluid were as follows: white blood cell count of 6,400 cells/mm3 with 97% of eosinophils (Fig. 2A) and red blood cell count of 12,800 cells/mm3; protein level, 4.7 g/dL; albumin level, 2.9 g/dL; and adenosine deaminase level, 20.2 U/L. Acid fast bacilli stain, bacterial culture and cytology of ascitic fluid were performed, but no specific findings were noted.
Echocardiography was performed to distinguish the cardiologic etiology of the ascites and cardiac involvement of eosinophilia, but there were no specific findings. A pulmonary function test was performed to exclude pulmonary involvement of eosinophilia. No specific findings were noted either. A gynecological evaluation was performed due to the elevated serum CA 125 level. No specific findings were observed. A colonoscopy was performed to explore the lesions of the distal ileum and colon. Esophagogastroduodenoscopy was also performed to exclude other malignancies. A diffuse edematous change of the ileocecal valve was noted on the colonoscopy, and a biopsy was performed (Fig. 3). In esophagogastroduodenoscopy, diffuse hyperemic mucosa was noted at the duodenum; a biopsy was also performed. The histopathology findings of a biopsy specimen in the terminal ileum and diffuse eosinophil infiltration in the lamina propria (over 40 eosinophils in high power field) were observed (Fig. 2B).
The patient was diagnosed with eosinophilic enteritis after excluding the malignancy, infections, and other rheumatoid diseases. The patient was treated with prednisolone 30 mg for eosinophilic enteritis. On the 2nd day of treatment, eosinophilia was improved in the peripheral blood (Fig. 4). From three days, improvement of abdominal bloating and abdominal distension was confirmed. The steroid therapy was tapered gradually for 4 weeks and stopped. The abdominal CT scan at 1 week after the end of the treatment showed the disappearance of ascites and improvement of bowel edema (Fig. 5). The patient was free of symptoms and she was followed up for 2 months.
EGID was diagnosed by the presence of gastrointestinal symptoms, histologically proven infiltration of eosinophil in one or more of the gastrointestinal tracts, and an absence of other causes of eosinophilia, such as parasite infection and malignancy.1 Eosinophilic gastroenteritis is a very rare disease (5.2/100,000 in the United States).4 Eosinophilic esophagitis is the most common among EGID, but enteritis or colitis is relatively rare. A range of symptoms are associated with the depth of the intestinal wall in eosinophilic enteritis.5 Diarrhea and abdominal pain are the main symptoms in the mucosal type while an obstruction is common in the muscular type. In the subserosal type, eosinophilic ascites and peripheral eosinophilia are common.
In the present case, abdominal pain and diarrhea, CT findings, peripheral eosinophilia, and eosinophilic ascites suggested eosinophilic enteritis. In addition, only an endoscopic biopsy was conducted and the mucosal type of eosinophilic enteritis was assumed. On the other hand, the present case is likely to be the subserosal type due to the presence of eosinophilic ascites and an elevation of the peripheral blood eosinophil count.7 Gastrointestinal symptoms can also be caused by an influenza A virus infection.8 In the present case, the histopathological findings were sufficient for a diagnosis of eosinophilic enteritis. Ascites have not been reported as a gastrointestinal symptom after an influenza A virus infection. Therefore, gastrointestinal symptoms can be considered symptoms associated with eosinophilic enteritis.
Eosinophilic enteritis is more frequent in allergic patients, and is usually caused by a food allergy.12 The present patient had a history of allergy to cats, but she had been in close contact with cats until recently. Since her abdominal symptoms occurred recently, the recent influenza infection might have triggered the eosinophilic enteritis. Chronic eosinophilic enteritis is accompanied by hypoalbuminemia and iron deficiency anemia.29 The patient's laboratory test showed no such findings. Therefore, her eosinophilic enteritis might have occurred recently. She had been isolated from her cat since she was hospitalized, and it took a week to diagnose her. In the meantime, there was no improvement in her symptoms. This suggests that eosinophilic enteritis is associated more with the recent influenza infection than with an allergic reaction to her cat. Peramivir, an influenza drug, may also be considered to be associated with eosinophilic enteritis, but there are no reports of an association between influenza and eosinophilic enteritis. In addition, the symptoms of eosinophilic enteritis, such as abdominal pain and diarrhea were present before the use of peramivir. Therefore, peramivir is unlikely to be the cause of eosinophilic enteritis.
A few studies have reported that a viral infection can act as a trigger for eosinophilic enteritis.1011 In a recent case, eosinophilic enteritis occurred after an influenza A infection in a child, but the patient had no known allergic disease.6 The mechanism through which influenza infection causes eosinophilic enteritis has not been elucidated, but after an influenza infection, the levels of various cytokines, such as interleukin (IL)-5 and IL-6, are increased.11213 IL-5 can induce eosinophil differentiation and proliferation.112 The increase in eosinophils due to IL-5 might have induced eosinophilic enteritis. Other studies have shown that elevated levels of IL-6 due to an influenza virus can stimulate eosinophils to induce pro-inflammatory effects.13 In the present case, there is a limit in explaining the precise mechanisms of eosinophilic enteritis because the cytokine levels could not be measured in a blood test. In addition, the presence of cytokines could not be confirmed by a histologic specimen. Another limitation is that it was difficult to explain the cause of the eosinophilic enteritis clearly. Moreover, it is currently unclear whether the influenza virus has exacerbated the existing allergy or has nothing to do with the existing allergy.
An elevation of the serum CA 125 level has been found in several cases. In the presence of ascites of an unknown cause, a malignancy or tuberculosis may be suspected.14 In the present case, cytology and acid fast bacilli staining of ascitic fluid were performed to exclude a malignancy and tuberculosis. On the other hand, no specific findings were noted. In a case report, an elevated CA 125 level was observed in eosinophilic enteritis with ascites.15 In that case report, although there was no mention of the cause of the elevated CA 125 level, the ratio of ascites/serum CA 125 and PET-CT were performed to exclude malignant ascites.14 Unfortunately, PET-CT was not performed due to problems of national health insurance and the patient's economic problems, and the ascitic CA 125 level was also not evaluated.
In the present case, gastrointestinal symptoms, peripheral eosinophilia, and ascites on abdominal CT were improved at the end of treatment and were maintained at the follow-up period. The patient has been observed without treatment because she is in good condition and preparing for pregnancy. In one case, the subserosal type of eosinophilic enteritis has a favorable response to steroid treatment with a single flare.9 In another case of eosinophilic enteritis, however, the rate of maintenance therapy is high if peripheral eosinophilia is present (absolute eosinophil count ≥1,500 cells/µL).3 The follow up period of the present patient was only two months. Therefore, continuous follow up will be needed.
In conclusion, the authors experienced a rare case of eosinophilic enteritis with eosinophilic ascites. To the best of the authors' knowledge, this is the first report of eosinophilic enteritis after an influenza A infection in a Korean adult with an allergy. The relationship between the eosinophilic enteritis and influenza A infection is inconclusive. Further research will be needed to determine the mechanism of eosinophilic enteritis.
Figures and Tables
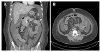 | Fig. 1Abdominal computed tomography showing a massive amount of ascites and diffuse edematous wall thickening at the ileum and ascending colon. (A) Coronal view. (B) Axial view. |
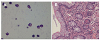 | Fig. 2Histologic images. (A) Most leukocytes in the ascitic fluid were observed as eosinophils (Wright stain, ×1,000). (B) Microscopic findings in a colonoscopic biopsy showing that numerous eosinophils are infiltrated in lamina propria (H&E, ×200). |
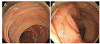 | Fig. 3Colonoscopy showing edematous and focally hyperemic mucosa at the (A) terminal ileum and (B) ileocecal valve. |
References
1. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113:11–28.

2. Pineton de Chambrun G, Dufour G, Tassy B, et al. Diagnosis, natural history and treatment of eosinophilic enteritis: a review. Curr Gastroenterol Rep. 2018; 20:37.

3. Lee J, Dierkhising R, Wu TT, Alexander J, Weiler C. Eosinophilic gastrointestinal disorders (EGID) with peripheral eosinophilia: a retrospective review at Mayo Clinic. Dig Dis Sci. 2011; 56:3254–3261.

4. Mansoor E, Saleh MA, Cooper GS. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol. 2017; 15:1733–1741.

5. Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore). 1970; 49:299–319.

6. Kwon JY, Huh JS, Je BK, Hong KD, Lee JH. Eosinophilic gastrointestinal disorder presenting as intractable vomiting and ascites in a young girl. Pediatr Gastroenterol Hepatol Nutr. 2017; 20:198–203.

7. Sheikh RA, Prindiville TP, Pecha RE, Ruebner BH. Unusual presentations of eosinophilic gastroenteritis: case series and review of literature. World J Gastroenterol. 2009; 15:2156–2161.

8. Minodier L, Charrel RN, Ceccaldi PE, et al. Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol J. 2015; 12:215.

9. Zhang M, Li Y. Eosinophilic gastroenteritis: a state-of-the-art review. J Gastroenterol Hepatol. 2017; 32:64–72.

10. Takeyama J, Abukawa D, Miura K. Eosinophilic gastroenteritis with cytomegalovirus infection in an immunocompetent child. World J Gastroenterol. 2007; 13:4653–4654.

11. Koga M, Fujiwara M, Hotta N, Matsubara T, Suzuki E, Furukawa S. Eosinophilic gastroenteritis associated with Epstein-Barr virus infection in a young boy. J Pediatr Gastroenterol Nutr. 2001; 33:610–612.

12. Terai M, Honda T, Yamamoto S, et al. Early induction of interleukin-5 and peripheral eosinophilia in acute pneumonia in Japanese children infected by pandemic 2009 influenza A in the Tokyo area. Microbiol Immunol. 2011; 55:341–346.

13. Sládková T, Kostolanský F. The role of cytokines in the immune response to influenza A virus infection. Acta Virol. 2006; 50:151–162.
14. Zhu FL, Ling AS, Wei Q, Ma J, Lu G. Tumor markers in serum and ascites in the diagnosis of benign and malignant ascites. Asian Pac J Cancer Prev. 2015; 16:719–722.

15. Hallal H, Más Mercader P, Alberca de las Parras F, et al. Eosinophilic gastroenteritis and CA-125 elevation. Gastroenterol Hepatol. 2004; 27:292–293.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download