Abstract
Background/Aims
The etiology of colon diverticulosis is related to a range of genetic, biological, and environmental factors, but the risk factors for asymptomatic diverticulosis of the colon are unclear. This study examined the risk factors for asymptomatic colon diverticulosis.
Methods
This retrospective study included examinees who underwent a colonoscopy for screening at the health check-up center of SAM Hospital between January 2016 and December 2016. The examinees with colon diverticulosis found by colonoscopy were compared with those without diverticulosis. The comparison factors were age, gender, alcohol consumption, smoking status, medical history, lipid profile, body mass index, visceral fat area, waist-hip ratio, and severity of a fatty liver.
Results
This study included 937 examinees and the overall prevalence of diverticulosis was 8.1% (76/937). Fatty liver was found in 69.7% (53/76) in cases of colon diverticulosis and 50.3% (433/861) in the control group (p=0.001). The average waist-hip ratio was 0.92±0.051 in colon diverticulosis and 0.90±0.052 in the control group (p=0.052). Multivariate analysis revealed the waist-hip ratio (OR=1.035, 95% CI 1.000–1.070, p=0.043), moderate fatty liver (OR=2.238, 95% CI 1.026–4.882, p=0.043), and severe fatty liver (OR=5.519, 95% CI 1.236–21.803, p=0.025) to be associated with an increased risk of asymptomatic colon diverticulosis.
Diverticulosis is the presence of one or more diverticula in the colon, which may be asymptomatic or symptomatic.1 Diverticular disease is symptomatic diverticulosis, including macroscopic diverticulitis, or symptomatic, uncomplicated diverticular disease, which has an absence of diverticulitis.1 Colonic diverticulosis is induced by a weakening of the supporting connective tissue and herniation of the mucosa and submucosa through the muscular layer of the colon wall.12 The prevalence of colon diverticulosis in Western society is less than 10% below the age of 40 and 50–66% over the age of 80.34 In Asia, diverticulosis is a rare disease, but the prevalence of colon diverticulosis in Asia has increased recently.56 In a recent study, the prevalence of diverticulosis in Korea was 12%.7 The reasons for the increasing prevalence of diverticulosis in Asia might be the westernized lifestyles affecting the pathogenesis of diverticulosis.78 The development of diverticulosis might be influenced by genetics, environmental factors, behavioral factors, and abnormal colonic wall motility.910 Approximately 10–30% of asymptomatic diverticulosis patients were reported to develop complications, such as diverticulitis and diverticular bleeding.11112
In previous studies, old age, alcohol, smoking, a high-fat diet, adenomatous polyps, and atherosclerotic disease were reported to be risk factors for asymptomatic colon diverticulosis.71314 On the other hand, the risk factors of asymptomatic colonic diverticulosis are not completely understood. Identifying the risk factors for asymptomatic diverticulosis is important because it can help prevent further complications, such as diverticular bleeding and diverticulitis. This study examined the characteristics of asymptomatic colon diverticulosis to identify the risk factors.
This retrospective study was approved by the Institutional Review Board of SAM Hospital (IRB number: 2018010). A retrospective study was conducted on 937 examinees who underwent a colonoscopy for screening at the health check-up center of SAM Hospital between January 2016 and December 2016. The inclusion criteria were as follows: 1) examinees older than 18 years and 2) asymptomatic examinees who underwent colonoscopy for a health checkup. The exclusion criteria were as follows: 1) prior history of colon surgery; 2) inflammatory bowel disease; and 3) the colonoscope could not be inserted into the cecum. Examinees with colon diverticulosis found on colonoscopy were compared with those without diverticulosis. The demographic characteristics, alcohol consumption, smoking status, and medical history were investigated through a self-answered questionnaire. The BMI, visceral fat area, and waist-hip ratio were measured using an Inbody 770 (Biospace Inc., Seoul, Korea).
Abdominal US was performed using the IU22 (Philips Healthcare, Bothell, WA, USA) scanner, with a 1-5 MHz convex abdominal transducer, by four experienced radiologists who had performed more than 1,800 abdominal US examinations. The technical parameters, such as depth, gain adjustment, placement of focal zone, and use of harmonics, were optimized on a case-by-case basis. All US images were evaluated independently on a picture archiving and communication system (ViewRex; TechHeim, Seoul, Korea). Fatty liver was diagnosed according to the difference in echogenicity between the kidney and liver, and visibility of the intrahepatic vessel walls and diaphragm.15 The severity of a fatty liver was classified into four classes according to the standard of a previous report: normal, mild, moderate and severe.15
Experienced gastrointestinal specialists, all of whom had performed more than 1,000 colonoscopies, conducted all examinations using a standard colonoscope (CF Q260AI; Olympus Optical Co., Ltd., Tokyo, Japan). All colonoscopies reached at least the cecum. Colon diverticulosis was classified as right colon diverticulosis, left colon diverticulosis, and bilateral colon diverticulosis according to the location.
Statistical analysis was performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). A Student's t-test was used to compare the continuous variables. The chi-squared test was used to compare the discrete variables. Logistic regression analysis was used to identify the risk factors for asymptomatic colonic diverticulosis. A p-value ≤0.05 was considered significant.
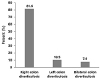
The total number of examinees was 937. The overall prevalence of asymptomatic colon diverticulosis was 8.1% (76/937). The prevalence of asymptomatic colon diverticulosis increased with age (Fig. 1). Examinees over 60 years old had the highest prevalence of asymptomatic colon diverticulosis, 10.8% (17/157). In asymptomatic colon diverticulosis, right, left, and bilateral colon diverticulosis was 81.6% (62/76), 10.5% (8/76), and 7.9% (6/76), respectively (Fig. 2). Among those examinees with asymptomatic colon diverticulosis, a single diverticulum and multiple diverticula was detected in 23.7% (18/76) and 76.3% (58/76), respectively.
In Table 1, the percentage of male and female asymptomatic colon diverticulosis was 76.3% (58/76) and 23.7% (18/76), respectively (p=0.056). The prevalence of asymptomatic colon diverticulosis in examinees older than 50 years (67.1%, 51/76) was higher than that in those younger than 50 years (32.9%, 25/76) (p=0.037). Hypertension was observed in 31.6% (24/76) of asymptomatic colon diverticulosis cases and 16.3% (140/861) of the control group (p=0.001). Diabetes mellitus, hyperlipidemia, smoking, and alcohol intake were similar in the asymptomatic colon diverticulosis and control groups. Fatty liver was noted in 69.7% (53/76) of asymptomatic colon diverticulosis group and 50.3% (433/861) in the control group (p=0.001). The following were observed in the asymptomatic colon diverticulosis group: normal livers (30.3%, 23/76), mild fatty livers (31.6%, 24/76), moderate fatty livers (32.9%, 25/76), and severe fatty livers (5.3%, 4/76). In the control group, there were normal livers (49.7%, 428/861), mild fatty livers (29.7%, 256/861), moderate fatty livers (19.3%, 166/861), and severe fatty livers (1.3%, 11/861). The triglyceride levels were 150.2±130.4 mg/dL in asymptomatic colon diverticulosis and 119.9±97.9 mg/dL in the control group (p=0.052). The waist-hip ratio was 0.92±0.05 and 0.90±0.05 in asymptomatic colon diverticulosis and control group, respectively (p=0.052).
The examinees with and without colon diverticulosis were classified into four different age groups (age <40, 40–49, 50–59, and ≥60 years). A higher portion of mild, moderate, and severe fatty liver was observed in examinees with diverticulosis than those without diverticulosis in three age groups (age <40, 40–49, and ≥60). The waist-hip ratio was also higher in the 40–49 year old examinees with diverticulosis than those without diverticulosis.
In Table 2, multivariate analysis revealed the waist-hip ratio (OR=1.035, 95% CI 1.000–1.070, p=0.043), moderate fatty liver (OR=2.238, 95% CI 1.026–4.882, p=0.043), and severe fatty liver (OR=5.519, 95% CI 1.236–21.803, p=0.025) to be associated with an increased risk of asymptomatic colon diverticulosis.
Colon diverticulosis is caused by a high intraluminal pressure and herniated mucosa through the muscular layers of the colon.1617 Genetic susceptibility, environmental factors, and abnormal colonic wall motility might be related to its development.910 The present results revealed the waist-hip ratio, moderate fatty liver, and severe fatty liver to be associated with an increased risk of asymptomatic colon diverticulosis. Several tools can be used to measure obesity, such as BMI, waist circumference, waist-hip ratio, and abdominal fat area. The BMI has limitations in measuring central obesity. Therefore, the waist-hip ratio, waist circumference, and abdominal fat area are more sensitive measurements for measuring central obesity than the BMI.1218 The present results revealed a moderate fatty liver and severe fatty liver to be significant risk factors for colon diverticulosis, but a previous study reported contrary findings regarding fatty liver.19 In that study, diverticulosis in the elderly (>65 years) was reported to be a negative predictor of hepatosteatosis.19 They examined elderly patients (>65 years) with diverticulosis. 19 In the present study, the examinees were older than 18 years old. The difference in age enrolled for each study might have caused the different results. The reasons why mild fatty liver was not related to colon diverticulosis could not be explained in this study. Moderate and severe fatty liver might be more related to colon diverticulosis than a mild fatty liver. Although there is no definite evidence to correlate asymptomatic diverticulosis with a fatty liver, it was hypothesized that altered microbiota might be associated with the mechanism linking colon diverticulosis and fatty liver. Several studies on the difference in fecal microbiota between healthy controls and asymptomatic colon diverticulosis have been reported.2021 Akkermansia muciniphila is more abundant in the fecal samples of people with asymptomatic colon diverticulosis than in healthy controls.20 A decrease in Clostridium cluster IV and microbiota with anti-inflammatory activity, such as Faecalibacterium prausnitzii, was observed in the asymptomatic diverticulosis group than the healthy controls.21 Therefore, the intestinal microbiota might play a role in the pathogenesis of diverticulum. An alteration of the intestinal microbiota might be also related to hepatic fat deposition and can affect the progression of fatty liver severity.2223 The present study regarding fatty liver, alcoholic hepatitis, and non-alcoholic fatty liver disease were not differentiated. Previous studies of the intestinal microbiota were performed on patients with non-alcoholic fatty liver disease.2223
Inconsistent results regarding visceral fat and asymptomatic diverticulosis have been reported. In the present results, visceral fat measured by Inbody 770 (Biospace Inc.) was not associated with asymptomatic diverticulosis. In other studies, however, visceral fat measured by CT was found to be associated with colonic diverticulosis.1618 A methodological difference was observed in measuring the visceral fat between the present study and previous studies.1618 The BMI was used to define obesity, but the BMI is not an exact indicator of central obesity. Inconsistent results regarding the BMI and colon diverticulosis have been reported.7918242526 BMI is a risk factor for colonic diverticulosis.926 In other reports, colonic diverticulosis was not related to BMI.7182425 As the waist-hip ratio and fatty liver were found to be associated with central obesity in this study, central obesity estimated by the waist-hip ratio and fatty liver might affect the pathogenesis of asymptomatic colon diverticulosis. How abdominal obesity contributes to the pathogenesis of diverticulosis is unclear. Two hypotheses regarding the relationship between diverticulosis and obesity have been proposed. First, methane gas produced by microbes in obese people might increase the colonic intraluminal pressure, which induces diverticulosis.16272829 Second, proinflammatory cytokines produced by adipocytes might affect the colonic motility related to colon diverticulosis.1630 On the other hand, the mechanism of the association between colonic diverticulosis and obesity has not been demonstrated. In the present study, high density lipoprotein, low density lipoprotein, and triglycerides were not associated with asymptomatic colon diverticulosis. In another report, there was no difference in the serum lipid profiles (total cholesterol, high density lipoprotein, low density lipoprotein and Triglycerides) between the control and diverticulosis groups,18 as in the present study. In this study, the prevalence of asymptomatic colon diverticulosis was higher in examinees over 50-years-old than in those younger than 50-years. The prevalence of colon diverticulosis increases with age.19133132 In this study, the prevalence of right colon diverticulosis was higher than that of left colon diverticulosis. The prevalence of left colon diverticulosis is higher in western society,33 whereas the prevalence of right colon diverticulosis is higher in Asian societies.7 Right colon diverticulosis in Asian people might be genetic and extends to left and bilateral colon diverticulosis in older age.34 On the other hand, the reason why right colon diverticulosis is predominant in Asian people is not completely understood. A difference in the humoral or neural system, sensitivity, and structural variation of the colon might be present.34
This study had several strengths. First, to the best of the authors' knowledge, this is the first study to investigate the relationship between the severity of fatty liver and asymptomatic colon diverticulosis. This study can provide an original aspect regarding the relationship between the severity of fatty liver and asymptomatic colon diverticulosis. Second, this study analyzed the parameters of obesity, such as lipid profile, visceral fat, waist-hip ratio, and BMI. Therefore, the association between obesity and diverticulosis was analyzed thoroughly. Third, to the best of the authors' knowledge, there has been no study on the relationship between the waist-hip ratio and asymptomatic diverticulosis. Instead of using the waist-hip ratio, there are reports showing that an increased waist circumference is related to colon diverticulosis.2435 On the other hand, this study has some limitations. First, this was a single-center retrospective study. Therefore, selection bias might be inevitable. Second, the number of examinees with diverticulosis enrolled was small (n=76). Therefore, care should be taken when generalizing these results to the entire population. Third, potential confounding factors for colonic diverticulosis such as the intake of meat and fiber, physical activity, and intake of other medications were not adjusted in this study. Fourth, age was not controlled in the analysis of the risk factors for colon diverticulosis.
In conclusion, the waist-hip ratio, moderate fatty liver, and severe fatty liver are risk factors for asymptomatic colon diverticulosis. Central obesity estimated by waist-hip ratio and fatty liver might affect the pathogenesis of asymptomatic colon diverticulosis. Further research will be needed to identify the risk factors for asymptomatic diverticulosis.
Figures and Tables
References
1. Strate LL, Modi R, Cohen E, Spiegel BM. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol. 2012; 107:1486–1493.

2. Spiller RC. Changing views on diverticular disease: impact of aging, obesity, diet, and microbiota. Neurogastroenterol Motil. 2015; 27:305–312.

4. Ye H, Losada M, West AB. Diverticulosis coli: update on a “Western” disease. Adv Anat Pathol. 2005; 12:74–80.
5. Nagata N, Niikura R, Aoki T, et al. Increase in colonic diverticulosis and diverticular hemorrhage in an aging society: lessons from a 9-year colonoscopic study of 28,192 patients in Japan. Int J Colorectal Dis. 2014; 29:379–385.

6. Kim SY, Kim YS, Kim HT, et al. A prospective study of factors influencing on the clinical characteristics of colonic diverticulosis. Korean J Gastroenterol. 2013; 62:97–103.

7. Song JH, Kim YS, Lee JH, et al. Clinical characteristics of colonic diverticulosis in Korea: a prospective study. Korean J Intern Med. 2010; 25:140–146.

8. Martel J, Raskin JB. History, incidence, and epidemiology of diverticulosis. J Clin Gastroenterol. 2008; 42:1125–1127.

9. Kopylov U, Ben-Horin S, Lahat A, Segev S, Avidan B, Carter D. Obesity, metabolic syndrome and the risk of development of colonic diverticulosis. Digestion. 2012; 86:201–205.

10. Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease--a twin study. Aliment Pharmacol Ther. 2012; 35:1103–1107.
11. Hjern F, Wolk A, Håkansson N. Obesity, physical inactivity, and colonic diverticular disease requiring hospitalization in women: a prospective cohort study. Am J Gastroenterol. 2012; 107:296–302.

12. Strate LL, Liu YL, Aldoori WH, Syngal S, Giovannucci EL. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology. 2009; 136:115–122.e1.

13. Nagata N, Niikura R, Shimbo T, et al. Alcohol and smoking affect risk of uncomplicated colonic diverticulosis in Japan. PLoS One. 2013; 8:e81137.

14. Wang FW, Chuang HY, Tu MS, et al. Prevalence and risk factors of asymptomatic colorectal diverticulosis in Taiwan. BMC Gastroenterol. 2015; 15:40.

15. Lee SH, Yun SJ, Kim DH, Jo HH, Park YS. Severity of nonalcoholic fatty liver disease on sonography and risk of coronary heart disease. J Clin Ultrasound. 2017; 45:391–399.

16. Nagata N, Sakamoto K, Arai T, et al. Visceral abdominal obesity measured by computed tomography is associated with increased risk of colonic diverticulosis. J Clin Gastroenterol. 2015; 49:816–822.

17. Burkitt D. Diverticular disease of the colon epidemiological evidence relating it to fibre-depleted diets. Trans Med Soc Lond. 1973; 89:81–84.
18. Lee SP, Ahn YW, Lee OY, Lee KN. The relationship between colonic diverticulosis and abdominal visceral and subcutaneous fat accumulation measured by abdominal CT scan. Turk J Gastroenterol. 2014; 25:192–197.

19. Sahin A, Tunc N, Demirel U, Kursat Poyrazoglu O, Yalniz M, Halil Bahcecioglu I. Relationship between diverticulosis and nonalcoholic fatty liver disease in elderly patients. J Int Med Res. 2018; 46:1545–1554.

20. Tursi A, Mastromarino P, Capobianco D, et al. Assessment of fecal microbiota and fecal metabolome in symptomatic uncomplicated diverticular disease of the colon. J Clin Gastroenterol. 2016; 50 Suppl 1:S9–S12.

21. Barbara G, Scaioli E, Barbaro MR, et al. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut. 2017; 66:1252–1261.

22. Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014; 20:16452–16463.

23. Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012; 18:2609–2618.

24. Teixeira C, Trabulo D, Ribeiro S, et al. Colonic diverticulosis and the metabolic syndrome: an association? Rev Esp Enferm Dig. 2017; 109:768–771.

25. Sharara AI, El-Halabi MM, Mansour NM, et al. Alcohol consumption is a risk factor for colonic diverticulosis. J Clin Gastroenterol. 2013; 47:420–425.

26. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012; 142:266–272.e1.

27. Mathur R, Amichai M, Chua KS, Mirocha J, Barlow GM, Pimentel M. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J Clin Endocrinol Metab. 2013; 98:E698–E702.

28. Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006; 290:G1089–G1095.

29. Weaver GA, Krause JA, Miller TL, Wolin MJ. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986; 27:698–704.

30. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007; 132:2169–2180.

31. Delvaux M. Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther. 2003; 18 Suppl 3:71–74.

32. Commane DM, Arasaradnam RP, Mills S, Mathers JC, Bradburn M. Diet, ageing and genetic factors in the pathogenesis of diverticular disease. World J Gastroenterol. 2009; 15:2479–2488.

33. Dore MP, Pes GM, Marras G, et al. Risk factors associated with colonic diverticulosis among patients from a defined geographic area. Tech Coloproctol. 2016; 20:177–183.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download