Hypertrophic osteopathy (HO) is a bone disorder of periosteal proliferation rendering a porous and irregular aspect to the surface of the bones affected. This condition has been described in dogs and horses [
12], and usually involves (bilaterally and symmetrically) the diaphysis of the appendicular bones. This condition is associated mainly with lung diseases (neoplastic or non-neoplastic), and is also known as pulmonary HO [
1]. HO in cats has been reported mainly in association with thoracic [
345] and extra-thoracic tumors [
67]. In addition, there is a report in a cat, in which the cause was unknown [
2]. On the other hand, congenital heart disease has been reported in conjunction with HO in humans [
89] and dogs [
10]. This paper describes the clinical and pathological features of a case of HO associated with a congenital interventricular septal defect of the heart in a cat.
A 3-year-old neutered female mixed-breed cat, which tested negative for antibodies of feline immunodeficiency virus (FIV) and for antigens of feline leukemia virus (FeLV) with the SNAP FIV/FeLV Combo (IDEXX Laboratories, USA), was referred for a clinical evaluation because of a 9-month history of lethargy, progressive inappetence, and dyspnea. The clinical examination revealed pale mucous membranes, a heart rate of 240 bpm (reference value: 120–240 bpm), heart murmur classified as grade 4 (1–5), and thoracic stertor. A cardiac Doppler ultrasound examination revealed a severe defect in the interventricular septum, as well as turbulent blood flow, mild interventricular communication, and moderate blood regurgitation between both ventricles. The left ventricular free-wall thickness was increased both in the diastole and systole, measuring 19.2 mm (14.97 ± 2.00) and 10.6 mm (8.92 ± 1.23), respectively. An increase in the left atrium wall thickness and moderate mitral valve regurgitation, as well as mild pulmonary valve regurgitation were observed. The symptoms were treated with furosemide (2.5 mg/kg once daily [SID]) and benazepril (0.25 mg/kg orally [PO] SID), and a clinical improvement of the respiratory distress was observed. After 9-month of treatment, progressive and diffuse hard thickening of the thoracic and pelvic limbs (severe in humerus, radius and ulna, and mild in femur and tibia) was observed. These changes caused a stiff gait and a decreased range of motion. Radiographic changes were observed in the periosteal region of the long bones involving the epiphysis, metaphysis, and diaphysis. These lesions were bilateral and symmetrical, and consisted of radiopaque thickening with a brush border or palisading aspect (
Fig. 1). Euthanasia was elected because of the worsening clinical condition and poor prognosis.
The necropsy revealed marked bilateral and symmetrical hard thickening of the limbs, which was most severe in the thoracic limbs (scapula, humerus, radius, ulna, carpus, and metacarpals) and moderate in the pelvic limbs (femur, patella, tibia, fibula, tarsus, and metatarsals) (
Fig. 2). On the cut surface of the bones, marked thickening of the cortical periosteum by spongy to solid bone and replacement of most of the medullary cavity of these bones were noted. A marked reduction in the size of the humeral condyles was also observed, with flattening of the humerus-radio-ulnar joint surface. The ventral surfaces of the thoracic vertebral bodies (T7 to T12) were also moderately thickened, hard, and ankylosed.
In addition to the bone lesions, a severe congenital cardiac interventricular defect was observed. The heart was enlarged, globular, and diffusely pale brown with linear multifocal white areas in the epicardium. The interventricular septal defect measured 1.2 cm in diameter and connected the right and left ventricles (
Fig. 3). Marked concentric hypertrophy of the right ventricle (RV) that exhibited a mild decrease in the cardiac chamber volume and moderate thickening of the papillary muscles with prominent trabeculae was observed. Moreover, moderate left ventricular and atrial lumen dilatation (eccentric hypertrophy) was noted, in addition to a focally extensive stellar and fibrous lesion above the mitral valve (jet lesion) and a mild dilation of the initial portion of the pulmonary trunk. The lungs were shiny, red, and exuded large amounts of translucent fluid on cut surface (edema). No other gross abnormalities were observed.
Samples of bone (femur, tibia, humerus, radius-ulna, vertebra, and rib) and other organs were collected and fixed in 10% neutral buffered formalin for 24 to 48 h. The bone samples were then decalcified in an 8% nitric acid solution for 24 h. All tissues were then processed routinely for histopathology, and stained with hematoxylin and eosin. Full bone segments and longitudinal sections of the humerus, radius, ulna, femur, and tibia were boiled, and then bleached by immersion in 10% hydrogen peroxide. After bleaching, the irregular and porous surface of the bones cortical was evident (
Fig. 4).
Microscopically, a severe replacement of cortical bone with reactive immature woven bone was observed in all analyzed fragments. In addition, the proliferation of trabecular bone in the periosteum, which was interspersed with multiple osteocytes, was noted. The RV of the heart had a focally extensive area of cardiomyocyte necrosis associated with a moderate neutrophilic inflammatory infiltrate, which extended from the endocardium to the myocardium (jet lesions associated to the cardiac interventricular defect). The lungs had severe and diffuse alveolar edema with multiple intra-alveolar macrophages, some containing hemosiderin in the cytoplasm (heart failure cells). No other microscopic abnormalities were noted.
HO associated with a congenital interventricular septal defect is diagnosed in cats mainly through the clinical, radiographic, ultrasonography, and pathological features. HO in dogs and domestic cats has been reported to be associated with intrathoracic neoplasms [
31112]. In the present case, however, a cardiac association was indicated because initially, a high interventricular communicating defect was detected in this cat. This had a clinical course of 9-month, which probably led to the subsequent periosteal bone proliferation, predominantly involving the long bones. Similar to the present case, congenital and acquired cardiac diseases have rarely been associated with the development of HO in dogs [
1013]. In addition, previous studies in humans have described interventricular communicating defects as the main cardiac condition associated with HO [
14].
Ventricular septal defect (VSD) in neonates often presents with eccentric right ventricular hypertrophy caused by a left-to-right cardiac shunt [
15]. In the present case, however, concentric RV hypertrophy was noted, which is likely the result of chronicity. This resulted in pulmonary hypertension, and possibly, shunt reversal, in which the heart flow may be reversed from the right to left chamber [
15], as evidenced by the blood regurgitation between both ventricles observed in the cardiac Doppler ultrasound examination. Therefore, concentric RV hypertrophy may have been caused by an increased afterload at the pulmonary trunk, whereas the eccentric left ventricle hypertrophy resulted both from the increased pulmonary preload, and probably from shunt reversal (right-to-left) [
15]. Furthermore, only left heart failure lesions were observed in this case; no ascites and nutmeg liver were observed. The theory of the authors is that compensatory mechanisms of the right heart, such as concentric cardiac hypertrophy, were effective in pumping the increased systolic loads, whereas the left ventricle compensatory mechanisms failed, resulting in severe and diffuse alveolar edema and heart-failure cells.
Clinically, the cat showed mild signs of lethargy, dyspnea, and pale mucous membranes, which comprised part of the clinical picture of heart failure. This is characterized mainly by exercise intolerance, ascites, dyspnea, syncope, and cyanotic mucosa [
15]. The treatment probably prolonged the cat's survival time and may have favored the development of HO. This was corroborated by previous studies in humans, which showed a higher frequency of HO associated with cardiac defects in individuals older than 6-year [
8]. Furthermore, HO is uncommon in young animals and more common in aged animals, in which neoplasia is more frequent [
1], and is often related to neoplasms [
3]. Increased blood flow to the limbs is a constant finding in HO, but the pathogenesis of the condition is still uncertain [
1]. In veterinary medicine, 3 main hypotheses have been proposed: a neurogenic theory, in which impulses originating in the thoracic lesions are conducted via the vagus nerve to the brainstem causing an initial reflex vasodilation; a humoral hypothesis, in which the primary thoracic lesion produces hormones or hormone-like substances; and the release of growth factors from platelets within the abnormal limb circulation [
1]. In the present case, the hypothesis was that the bone proliferative lesions were caused by the release of growth factors, similar to the pathogenesis observed in humans [
812]. In this species, HO may have been related to the presence of substances in the bloodstream, such as reduced ferritin and prostaglandin E, which, under physiological conditions, would be inactivated or removed by the lungs [
8]. In the present case, however, the secondary severe pulmonary involvement may have reduced the efficiency of this clearance process. This may have provided the release of growth factors and later the development of HO. In addition, there was no evidence of an inflammatory process in the bone samples.
HO is a bone proliferative disorder in cats, and is commonly associated with intrathoracic or extrathoracic neoplasms [
345712]. Microscopically, the bone proliferative changes consisted of an intense replacement of cortical bone by reactive immature woven bone in addition to the proliferation of periosteal connective tissue. Neoplastic and inflammatory cells were not observed in the lesions, which allowed the condition to be differentiated from other causes of proliferative appendicular bone disorders in cats, such as polyarthritis, degenerative joint disease, inflammatory synovitis, bacterial and fungal osteomyelitis, and mesenchymal neoplasms [
1].
HO typically presents with new bone formation that initially affects the limb bones, extending proximally [
1]. In the present report, the periosteal bone hyperostosis was diffuse and severe both in the proximal and distal limb bones, but this did not involve the phalanges. In addition, moderate thickening of the thoracic vertebral bodies (T7 to T12) was observed, which was distinct from that described previously, in which bone lesions associated with HO are limited to the appendicular bones [
12]. Congenital abnormalities, such as an interventricular septal defect observed in the present case, may lead to the development of HO in cats. The bone proliferative lesions might have been more severe as a collective result of a VSD, pulmonary edema, and myocardial necrosis. These probably aggravated the hypoxia in this cat, and most likely affected the most extensive distribution of the lesions.
Figures and Tables
Fig. 1
Hypertrophic osteopathy in a cat. Craniocaudal radiography showing marked radiopaque thickening in the periosteal region.
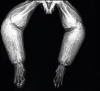
Fig. 2
Hypertrophic osteopathy in a cat. On gross examination of the cat, there was a bilaterally symmetrical increase in limb thickness, which was severe in the forelimbs and moderate in the pelvic limbs.

Fig. 3
Gross examination of the heart. Globular-looking heart with a pale color, showing interventricular septal defect, 2 cm in diameter, which connected the right and left ventricles.
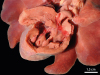
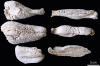
Fig. 4
Hypertrophic osteopathy in a cat. Boiled and bleached segments and humeral cuts of the humerus, radius and ulna, scapula, tibia and femur (arranged in a counterclockwise direction beginning at the top left corner), showed marked thickening of the cortical bone, with an irregular, variably solid to porous surface.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download