This article has been
cited by other articles in ScienceCentral.
Abstract
In ultrasound/computed tomography (CT) fusion images, ultrasound allows visualization of the target in real time. CT provides a navigation for ultrasound scanning and improves the overview in areas of limited visualization with ultrasound. This study was performed to investigate the feasibility of ultrasound/CT fusion based on an electromagnetic tracking technique using external fiducial markers for canine ocular and periocular regions. In 7 Beagle dogs, contrast-enhanced CT images of the head were obtained with placing external fiducial markers over the frontal region and both sides of the forepaws of the dog. Ultrasonography was performed under a magnetic field by installing a position sensor in the linear probe, without changing the dog's position. The positions of the external fiducial markers were adjusted and matched, based on the CT images. The execution time of co-registration and the distance between the regions of interest and the co-registration points, the frontal bone, cornea, retina, and optic nerve, were estimated. Approximately 60% of external fiducial markers were properly recognized in all dogs. After adjustment, all external fiducial markers were precisely matched. The co-registration execution time was less than 1 min. The distances between the regions of interest and co-registration points were less than 3 mm in all dogs. The electromagnetic tracking technique using external fiducial markers was a simple and applicable method for fusion imaging of a canine head using real-time ultrasonography and CT. This technique can be useful for interventional procedures of retrobulbar and periorbital lesions.
Keywords: Co-registration, electromagnetic tracking technique, external fiducial markers, ocular and periocular, ultrasound/CT fusion
INTRODUCTION
Various diagnostic imaging modalities are widely used in veterinary medicine, including radiography, ultrasonography, computed tomography (CT), and magnetic resonance imaging. Fusion imaging is a multimodal technology that allows merging of image data obtained from 2 identical or different modalities to form a composite image. Fusion imaging can improve understanding of the features of the target lesion because each imaging modality has different inherent imaging properties for the same lesion. Fusion imaging can also improve the accuracy when conducting interventional procedures, particularly for small target lesions [
1]. Historically, anatomic imaging modalities, superior for the depiction of anatomic information, have been combined with metabolic functional imaging such as positron emission tomography and single photon emission computerized tomography as fusion imaging in both human and veterinary medicine [
2]. Although there has been no study about ultrasound/CT fusion imaging in veterinary medicine, fusion imaging of ultrasound and CT has been widely applied in humans in the previous two decades to improve the lesion conspicuity to provide reliable and accurate guidance for biopsy or interventional procedures, and to increase the feasibility of radiofrequency ablation for lesions that are difficult to access due to their small size or that are inconspicuous on ultrasound [
34]. In ultrasound/CT fusion images, ultrasound allows visualization of the target in real time; CT provides a navigation for ultrasound scanning and improves the overview in areas of limited visualization with ultrasound, such as lesions containing air or that are located behind air or bone. Thus, ultrasound/CT fusion images have been applied to various targets including the liver, spleen, kidney, breast, brain, and musculoskeletal system [
56].
In ultrasound/CT fusion imaging of the target, precise registration between 2 modalities (co-registration) and the time required for image fusion are important. Co-registration can be performed by intrinsic registration using optical tracking, image-based tracking using vascular intervention, or landmark alignment, and by extrinsic registration using artificial electromagnetic tracking [
67].
In electromagnetic tracking, a magnetic field generator creates a magnetic field and induces currents in the position sensor mounted on the transducer. Image fusion between the ultrasound and CT can be achieved automatically using external fiducial markers containing position sensor coils. The time required for image fusion depends on the equipment technique and the operator's experience. Electromagnetic tracking reduces the time required for image fusion by simplifying the co-registration procedure, and is helpful for less experienced operators compared to manual registration [
8].
As the availability of advanced imaging modalities in veterinary medicine increases, ultrasound/CT fusion imaging is expected to be used for diagnosis and intervention. This study was performed to assess the feasibility of ultrasound/CT fusion, based on electromagnetic tracking using electromagnetic fiducials in canine ocular and periocular regions.
MATERIALS AND METHODS
Seven intact male purpose-bred Beagles were used in this study. The median age of the dogs was 3 years (2–4 years) and the median weight was 9.9 kg (7.0–12 kg). All dogs were healthy based on a physical examination, complete blood count, biochemistry, and radiography. Dogs were individually housed in cages and were fed commercial dry food and water ad libitum. The study protocol was approved by the Institutional Animal Care and Use Committee at Chonnam National University. The protocol for the care of dogs adhered to the Guidelines for Animal Experiments of Chonnam National University (CNU IACUC-YB-2017-55).
After fasting for 12 h, general anesthesia was induced by intramuscular injection of 0.75 mg/kg zolazepam hydrochloride-tiletamine hydrochloride (Zoletil; Virbac, France) and 0.03 mg/kg medetomidine hydrochloride (Domitor; Orion Corporation, Finland), and was maintained with isoflurane (1%–2%) and oxygen (1 L/min).
The procedure was performed on the CT table; the ultrasound machine (Epiq7; Philips Healthcare, Netherlands) and magnetic field generator were positioned next to the table. Each dog was positioned in ventral recumbency with the forelimbs extended cranially. After clipping the hair, 3 sterile registration patches, each containing 2 fiducials, were placed on the skin over the forehead and the dorsal part of the left and right carpi (
Fig. 1A). The registration patches were temporarily sutured to the skin to prevent movement.
Fig. 1
Ultrasound/CT fusion imaging of a dog. (A) After positioning the dog on the CT table in dorsoventral recumbency and extending the forelimbs cranially, each set of 2 electromagnetic registration fiducials were attached over the forehead and the dorsal part of both the left and right carpi. The ultrasound machine and magnetic field generator were positioned next to the dog. (B) The ultrasound sensor coil tracker was mounted on the linear probe.
CT, computed tomography.

A pre-contrast CT scan was performed using a 16-row multidetector CT scanner (Siemens Emotion 16; Siemens AG, Germany) with the following settings: slice thickness, 1.5 mm; pitch, 0.65; rotation duration, 1,000 ms; tube voltage, 130 kV; tube current, 120 mA. The field of scan view was from the nostril to the third cervical vertebra. Contrast CT images were collected approximately 30 sec after manual injection of 2 mL/kg iohexol (Omnihexol 300; Korea United Pharm Co., South Korea).
The ultrasound sensor coil tracker (Philips Healthcare) was mounted on the 5–12 MHz linear probe; the electromagnetic field generator was positioned near the dog and the registration fiducials (
Fig. 1B). The enhanced CT images were uploaded to the ultrasound machine by the hospital network. After running the installed software (PercuNav; Philips Healthcare), the registration fiducials were automatically identified, and co-registration of CT and ultrasonography to achieve combined images was accomplished using the registration fiducials. If the external fiducials were not automatically registered or were mismatched, manual registration adjustment was performed by the radiologist (J.H.C.).
Once the images were fused, semi-transparently overlaid ultrasound and CT images were scanned in real time, allowing visualization of the ocular and periocular areas in each ultrasound and CT image. The co-registration error was defined as the distance between the regions of interest (the frontal bone, cornea, retina, and optic nerve) and the co-registration points displayed on the CT image. The minimum and maximum distances were measured. The time necessary for complete co-registration and the co-registration error between ultrasonography and CT images using the external electromagnetic fiducials were estimated.
RESULTS
Successful fusion of real-time ultrasound scans with pre-acquired CT images was achieved for all dogs. The position and orientation of the transducer were provided by an electromagnetic tracking system using a sensor mounted on the probe, and allowed the examiner to understand the target and the scan plane. The previously achieved CT images were transferred to the ultrasound system by the hospital network, and at least 60% of the external electromagnetic markers were automatically detected in each dog. The verification and adjustment of the markers by the examiner were easy and took little time. After adjustment, all external fiducial markers were precisely matched. The co-registration time for each dog was less than 1 min.
After registration, the CT images were projected by fitting them side by side with the ultrasound image. During the ultrasound scanning of the target, the reformatted planes of the CT series were continuously generated according to the imaging planes of the ultrasound transducer. The reformatted planes of the CT images were displayed side by side with, and also overlaid on, the ultrasound image. Therefore, the examiner could interpret and compare the ultrasound with the reference CT image by visualizing the target from the same angle (
Fig. 2). In addition, the ultrasound scan angle was displaced over the volume-rendering CT images. The grey shade of the fusion image was controlled to emphasize the structure visualized on either ultrasonography or CT images.
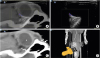
Fig. 2
Fusion image (A) of the ocular and periocular areas co-registered with ultrasonography (B) and CT (C) and in a Beagle. (D) The gray box on the volume-rendered CT image marks the scanned area and displays the scan angle. Blood flow in the posterior segment of the globe is invisible on color Doppler ultrasonography and overlaid the fusion image.
CT, computed tomography.
The lens, ciliary body, retina, optic nerve, periocular muscle, and border of the orbit were displayed side by side in the ultrasound and CT images. The intraocular structures including the ciliary body, iris, vitreous chamber, optic disk, and anterior chamber were only identified in ultrasound images with high resolution. The retrobulbar muscle, orbit, and fascial skeletal structures were clearly observed on contrast-enhanced CT images. Fusion images clarified the space between the eyeball and orbit and distinguished the hypoechoic optic nerve from the contrast-enhanced retrobulbar muscles (
Fig. 3). Blood flow of the anterior and posterior segment of the globe and the retrobulbar region could be overlaid CT image and investigated on the fusion image real time. The distance between the regions of interest and the co-registration points was < 3 mm in all dogs. The minimum distance between the points was 0 from all regions of interest; the maximum distance was 3 mm.
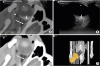
Fig. 3
Fusion images of the ocular and periocular areas in a dog. (A) Fusion images, (B) CT, (C) ultrasound images, and (D) reconstructed volume-rendered CT images. The lens (*), eyeball (arrow head), optic nerve (short arrow), periocular muscle (long arrow), and border of the orbit were displayed side by side in both CT and ultrasound images. Fusion images clarified the space between the eyeball and orbit and distinguished the hypoechoic optic nerve from the contrast-enhanced retrobulbar muscles.
CT, computed tomography.
DISCUSSION
This study described the feasibility of image fusion between CT scanning and ultrasound using electromagnetic tracking of the ocular and periocular regions in dogs. The ultrasound images were overlaid on previously acquired CT images during real-time ultrasound scanning of the ocular and periocular areas, using the CT images as references. The periocular area provided a limited acoustic window for ultrasound. The ocular and periocular areas had low tissue contrast for CT studies. The lens, eyeball, optic nerve, periocular muscle, and border of the orbit were seen in each image obtained by ultrasound and enhanced CT images. However, intraocular structures including the ciliary body, iris, vitreous chamber, optic disk, and anterior chamber were only visualized in ultrasound images with high resolution. Contrast-enhanced CT images described the retrobulbar muscle, orbit, and fascial skeletal structures in detail. The ultrasound/CT fusion images provided image detail of the ocular and periocular areas and the examiner could understand and compare the characteristic findings of the each structure because the ultrasound and CT images were shown side by side after co-registration. Real-time fusion imaging is limited by the high costs of the systems and the need for supplementary time for image fusion including registration [
6]. The reliability and accuracy of co-registration between the combined imaging modalities and the decreased time required for image fusion are critical for the clinical application of this technique. Ultrasound images have been fused to images of other modalities using optical, infrared, or electromagnetic tracking [
9]. When using internal anatomic landmarks, the vessel or the configuration of the target can be used for registration. In our prior experiments, the internal anatomic landmarks were not suitable for the ocular and periocular areas, as blood vessels are not plentiful in this area and the configuration of the eye could not specify the image plane or point. The time necessary for image fusion using internal anatomic landmarks could not be determined due to lack of study in veterinary medicine however 3 experienced examiners performed registration in less than 5 min in human medicine [
6]. The extrinsic electromagnetic tracking system simplified the registration, increased the accuracy of multimodality fusion, and enabled a wider use of fusion imaging by examiners with less experience [
8]. In our pilot test about a manual registration in fusion imaging, manual registration using surface or vessel navigation was not easy for operators without extensive experience and this method required more time for co-registration than electromagnetic tracking. In our study, electromagnetic tracking could adjust the imaging points after the initial plane-lock using the electromagnetic fiducials. Three fiducials were placed in a triangle over the forehead and bilateral paws, with the target in the center. Most of the electromagnetic fiducials were automatically identified in all dogs, and even the mismatched or undetected fiducials could be manually set using a simple process. Thus, the co-registration time was less than 1 min.
Organ deformation and distortion by respiration, patient movement, or differences in positioning between examinations also affect the quality of fusion imaging and the time required for the procedure [
6]. In this study, CT images of the skull of each dog in a dorsoventral position were scanned on a CT table to facilitate attachment of the fiducials, and an ultrasound scan of the ocular and periocular regions was acquired to prevent altering the dog's position. However, probe compression induced changes in the position of the target during ultrasound scanning. Because the pre-acquired CT images are “rigid images,” a larger target deformation in real-time ultrasound scanning caused a greater discrepancy between the CT and ultrasound images. In this study, the skeletal structures around the ocular and periocular areas were less vulnerable to probe compression. In our pilot study for fusion imaging of the liver and spleen, probe compression over the abdominal wall had a greater effect on matching between the CT and ultrasound images than the ocular and periocular areas. Unlike human medicine, anesthesia is required for CT scanning in most dogs and the liver and spleen tended to be more susceptible to probe compression due to the reduction of abdominal pressure. The eye movement was also considered in the present study because fusion images of the ocular and periocular regions was made. In this study, general anesthesia was induced using medetomidine and zolazepam-tiletamine and maintained with isoflurane. The anesthetic depth was enough to reduce the eye movement in the dog between the CT and ultrasound images [
10]. Respiration was another primary cause of difficulties in fusion imaging related to the liver and spleen. Previous human studies of fusion imaging of the liver used the electromagnetic tracking device for co-registration between ultrasound and CT or magnetic resonance imaging instead of the internal tracking method. However, even when using electromagnetic tracking, the average registration error of liver fusion images was approximately 8 mm [
11121314]. Further studies should investigate the scan window which allows less compression in an ultrasound scan, to determine reliable image fusion for each abdominal organ in dogs.
In human medicine, fusion imaging has been primarily used for abdominal organs. Only one study was about intraoperative ultrasound scanning of the brain with CT fusion for tumor detection in neurosurgery and 2 studies of musculoskeletal ultrasound/CT fusion imaging were performed in cadavers or patients suffering from sacroiliitis, osteoarthritis, and rheumatoid arthritis [
151617]. In all 3 studies, ultrasound/CT fusion imaging was performed using the electromagnetic tracking device with high accuracy. To the authors' knowledge, the present study is the first report of fusion imaging of the ocular and periocular areas. The high success rate of automatic matching and the simple adjustment of external electromagnetic fiducials, short co-registration time, and high accuracy of image fusion indicated that fusion imaging could be useful for the investigation of various pathologies involving ocular and periocular lesions, including tumors, cellulitis, and abscesses as well as sampling and intervention for the retrobulbar lesions and lesions between the eye and the orbit.
There are some limitations of this study. Fusion imaging was conducted only in normal dogs; thus, the clinical usefulness of fusion imaging should be investigated further in dogs with ocular and periocular diseases. A small number of subjects of the same breed were used. The automatic recognition of fiducials and successful matching rate may differ when applied to a breed with a different skull shape and size. Inter- and intra-observer variations were not estimated. In spite of these limitations, this study has clinical significance: fusion imaging between ultrasonography and CT was introduced in veterinary medicine for the first time. The technique is an advance in the field of diagnostic imaging and can be applied to various diseases in dogs.
In this study, fusion imaging of real-time ultrasound with CT of the ocular and periocular area was successfully conducted using electromagnetic fiducials, with acceptable registration errors for CT-ultrasound registration. Ultrasound/CT fusion imaging could provide spatial information and real-time imaging of canine ocular and periocular areas. Fusion imaging using external electromagnetic fiducials can be applied to veterinary patients with ocular and periocular lesions that are challenging to diagnose, and to interventional procedures to improve accuracy and safety.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download