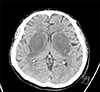
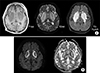
A 47-year-old man with diabetes mellitus, who had been undergoing maintenance hemodialysis for the past six months, visited the emergency room due to deterioration of consciousness and dysarthria. Type 2 diabetes had been diagnosed 10 years ago and antidiabetic agents, including gemigliptin and gliclazide, were not prescribed 3 months ago due to hypoglycemic episodes during hemodialysis. His vital signs were stable. His laboratory values were as follows: hemoglobin, 10.9 g/dL; blood urea nitrogen, 62.8 mg/dL; serum creatinine, 6.28 mg/dL; sodium, 140 mEq/L; HbA1c, 5.6%; glucose, 65 mg/dL; PTH, 88.0 pg/mL; and HCO3−, 17.1 mM/L. The values of hemoglobin and spKt/V during the last 6 months were stable. Dysarthria and rigidity of extremities were observed. Computed tomography showed hypodense lesions in bilateral basal ganglia (Fig. 1). T2-weighted magnetic resonance imaging revealed high signal intensity in the basal ganglia and T2-FLAIR showed hyperintensity accompanied by the lentiform fork sign (LFS). T1-weighted imaging revealed low signal intensity on the basal ganglia (Fig. 2A). LFS presents in conditions, such as metabolic acidosis, uremia, and hyperosmolar hyperglycemic states. Diffusion weighted imaging has also showed a rim-shaped diffusion restriction around the periphery of the basal ganglia (Fig. 2B).
Uremic encephalopathy (UE), which is rarely developed, presents with clinical manifestations including headaches, dysarthria, and seizures.1 The pathological mechanism underlying UE is unclear, however, several factors, such as fluctuation in blood glucose, uremia, and metabolic acidosis, seem to be essential for UE. The lesions from UE usually occur in the cortical or subcortical regions of the parietooccipital area. An involvement of the basal ganglia is uncommon in uremic patients.2 However, the basal ganglia are vulnerable to uremic toxins, particularly in diabetic patients due to endothelial dysfunction.3 Also, focal deficit of glucose can induce bilateral basal ganglia lesions in diabetic uremia.45 Therefore, uncontrolled hyperglycemia or recurrent hypoglycemia can cause acute basal ganglia lesions.5 Vasculitis, which causes endothelial damage, can often cause bilateral basal ganglia lesions, but this patient had only hypoglycemic episodes without inflammatory signs.6 The basal ganglia lesions caused by cytotoxic edema in uremia usually show specific findings, such as LFS or diffusion restriction, compared with other neurologic diseases.7 We assume that significant hypoglycemia and uremia caused the bilateral basal ganglia lesions in this case. The neurologic symptoms of the patient did not improve despite intensive hemodialysis for several months. Follow-up EEGs still showed slow waves. Thus, the prevention of hypoglycemia in uremic patients can be a good option for preventing UE.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download